Toxicology and Applied Pharmacology ( IF 3.8 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.taap.2020.114958 Moemi Kawaguchi 1 , Takumi Nukaga 2 , Shuichi Sekine 1 , Akinori Takemura 1 , Takeshi Susukida 1 , Shiho Oeda 2 , Atsushi Kodama 2 , Morihiko Hirota 2 , Hirokazu Kouzuki 2 , Kousei Ito 1
|
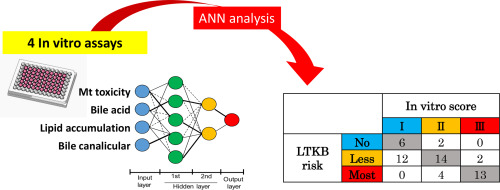
Drug-induced liver injury (DILI) can cause hepatic failure and result in drug withdrawal from the market. It has host-related and compound-dependent mechanisms. Preclinical prediction of DILI risk is very challenging and safety assessments based on animals inadequately forecast human DILI risk. In contrast, human-derived in vitro cell culture-based models could improve DILI risk prediction accuracy. Here, we developed and validated an innovative method to assess DILI risk associated with various compounds. Fifty-four marketed and withdrawn drugs classified as DILI risks of “most concern”, “less concern”, and “no concern” were tested using a combination of four assays addressing mitochondrial injury, intrahepatic lipid accumulation, inhibition of biliary network, and bile acid accumulation. Using the inhibitory potencies of the drugs evaluated in these in vitro tests, an algorithm with the highest available DILI risk prediction power was built by artificial neural network (ANN) analysis. It had an overall forecasting accuracy of 73%. We excluded the intrahepatic lipid accumulation assay to avoid overfitting. The accuracy of the algorithm in terms of predicting DILI risks was 62% when it was constructed by ANN but only 49% when it was built by the point-added scoring method. The final algorithm based on three assays made no DILI risk prediction errors such as “most concerned “ instead of “no concern” and vice-versa. Our mechanistic approach may accurately predict DILI risks associated with numerous candidate drugs.
中文翻译:

基于机制的集成化验系统,用于预测药物性肝损伤。
药物性肝损伤(DILI)可能会导致肝功能衰竭,并导致药物退出市场。它具有与宿主相关和与化合物相关的机制。临床上对DILI风险的预测非常具有挑战性,基于动物的安全性评估不足以预测人类DILI风险。相比之下,基于人的体外细胞培养模型可以提高DILI风险预测的准确性。在这里,我们开发并验证了一种创新的方法来评估与各种化合物相关的DILI风险。使用针对线粒体损伤,肝内脂质蓄积,胆道网络抑制和胆汁的四种测定方法的组合,对分类为DILI的“最关注”,“较少关注”和“无关注”风险的54种上市和退出药物进行了测试。酸积累。利用在这些体外测试中评估的药物的抑制力,通过人工神经网络(ANN)分析建立了具有最高可用DILI风险预测能力的算法。它的总体预测准确性为73%。我们排除了肝内脂质蓄积测定,以避免过度拟合。在用人工神经网络构建时,就预测DILI风险而言,该算法的准确性为62%,而在通过加分计分法构建时,仅为49%。基于三种分析的最终算法没有发生DILI风险预测错误,例如“最关注”而不是“无关”,反之亦然。我们的机制方法可以准确预测与多种候选药物相关的DILI风险。通过人工神经网络(ANN)分析,建立了具有最高可用DILI风险预测能力的算法。它的总体预测准确性为73%。我们排除了肝内脂质蓄积测定,以避免过度拟合。在用人工神经网络构建时,就预测DILI风险而言,该算法的准确性为62%,而在通过加分计分法构建时,仅为49%。基于三种分析的最终算法没有发生DILI风险预测错误,例如“最关注”而不是“无关”,反之亦然。我们的机制方法可以准确预测与多种候选药物相关的DILI风险。通过人工神经网络(ANN)分析,建立了具有最高可用DILI风险预测能力的算法。它的总体预测准确性为73%。我们排除了肝内脂质蓄积测定,以避免过度拟合。在用人工神经网络构建时,就预测DILI风险而言,该算法的准确性为62%,而在通过加分计分法构建时,仅为49%。基于三种分析的最终算法没有发生DILI风险预测错误,例如“最关注”而不是“无关”,反之亦然。我们的机制方法可以准确预测与多种候选药物相关的DILI风险。在用人工神经网络构建时,就预测DILI风险而言,该算法的准确性为62%,而在通过加分计分法构建时,仅为49%。基于三种分析的最终算法没有发生DILI风险预测错误,例如“最关注”而不是“无关”,反之亦然。我们的机制方法可以准确预测与多种候选药物相关的DILI风险。在用人工神经网络构建时,就预测DILI风险而言,该算法的准确性为62%,而在通过加分计分法构建时,仅为49%。基于三种分析的最终算法没有发生DILI风险预测错误,例如“最关注”而不是“无关”,反之亦然。我们的机制方法可以准确预测与多种候选药物相关的DILI风险。



























 京公网安备 11010802027423号
京公网安备 11010802027423号