当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyplex transfection from intracerebroventricular delivery is not significantly affected by traumatic brain injury.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.jconrel.2020.03.025 David J Peeler 1 , Nicholas Luera 1 , Philip J Horner 2 , Suzie H Pun 1 , Drew L Sellers 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.jconrel.2020.03.025 David J Peeler 1 , Nicholas Luera 1 , Philip J Horner 2 , Suzie H Pun 1 , Drew L Sellers 1
Affiliation
|
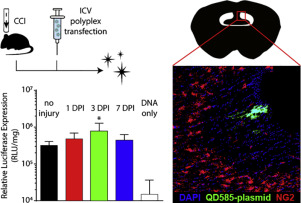
Traumatic brain injury (TBI) is largely non-preventable and often kills or permanently disables its victims. Because current treatments for TBI merely ameliorate secondary effects of the initial injury like swelling and hemorrhaging, strategies for the induction of neuronal regeneration are desperately needed. Recent discoveries regarding the TBI-responsive migratory behavior and differentiation potential of neural progenitor cells (NPCs) found in the subventricular zone (SVZ) have prompted strategies targeting gene therapies to these cells to enhance neurogenesis after TBI. We have previously shown that plasmid polyplexes can non-virally transfect SVZ NPCs when directly injected in the lateral ventricles of uninjured mice. We describe the first reported intracerebroventricular transfections mediated by polymeric gene carriers in a murine TBI model and investigate the anatomical parameters that dictate transfection through this route of administration. Using both luciferase and GFP plasmid transfections, we show that the time delay between injury and polyplex injection directly impacts the magnitude of transfection efficiency, but that overall trends in the location of transfection are not affected by injury. Confocal microscopy of quantum dot-labeled plasmid uptake in vivo reveals association between our polymers and negatively charged NG2 chondroitin sulfate proteoglycans of the SVZ extracellular matrix. We further validate that glycosaminoglycans but not sulfate groups are required for polyplex uptake and transfection in vitro. These studies demonstrate that non-viral gene delivery is impacted by proteoglycan interactions and suggest the need for improved polyplex targeting materials that penetrate brain extracellular matrix to increase transfection efficiency in vivo.
中文翻译:

脑室内分娩的多路体转染不受脑外伤的明显影响。
颅脑外伤(TBI)在很大程度上是无法预防的,通常会导致其受害者死亡或永久性致残。由于目前对TBI的治疗仅能改善初始损伤的继发效应,例如肿胀和出血,因此迫切需要诱导神经元再生的策略。在脑室下区域(SVZ)中发现的有关TBI反应性迁徙行为和神经祖细胞(NPC)分化潜能的最新发现,已促使针对这些细胞进行基因治疗的策略增强了TBI后的神经发生。先前我们已经表明,质粒多态复合物可以直接注射到未受伤小鼠的侧脑室中,从而可以非病毒转染SVZ NPC。我们描述了由鼠TBI模型中的聚合基因载体介导的首次报道的脑室内转染,并研究了通过这种给药途径决定转染的解剖学参数。使用萤光素酶和GFP质粒转染,我们显示损伤和多聚体注射之间的时间延迟直接影响转染效率的大小,但转染位置的总体趋势不受损伤影响。体内量子点标记质粒摄取的共聚焦显微镜显示我们的聚合物与SVZ细胞外基质带负电的NG2硫酸软骨素蛋白聚糖之间的关联。我们进一步验证了多聚体体外吸收和转染需要糖胺聚糖而不是硫酸盐基团。
更新日期:2020-03-19
中文翻译:

脑室内分娩的多路体转染不受脑外伤的明显影响。
颅脑外伤(TBI)在很大程度上是无法预防的,通常会导致其受害者死亡或永久性致残。由于目前对TBI的治疗仅能改善初始损伤的继发效应,例如肿胀和出血,因此迫切需要诱导神经元再生的策略。在脑室下区域(SVZ)中发现的有关TBI反应性迁徙行为和神经祖细胞(NPC)分化潜能的最新发现,已促使针对这些细胞进行基因治疗的策略增强了TBI后的神经发生。先前我们已经表明,质粒多态复合物可以直接注射到未受伤小鼠的侧脑室中,从而可以非病毒转染SVZ NPC。我们描述了由鼠TBI模型中的聚合基因载体介导的首次报道的脑室内转染,并研究了通过这种给药途径决定转染的解剖学参数。使用萤光素酶和GFP质粒转染,我们显示损伤和多聚体注射之间的时间延迟直接影响转染效率的大小,但转染位置的总体趋势不受损伤影响。体内量子点标记质粒摄取的共聚焦显微镜显示我们的聚合物与SVZ细胞外基质带负电的NG2硫酸软骨素蛋白聚糖之间的关联。我们进一步验证了多聚体体外吸收和转染需要糖胺聚糖而不是硫酸盐基团。









































 京公网安备 11010802027423号
京公网安备 11010802027423号