当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Yeast Two-Hybrid Screening of Photoswitchable Protein-Protein Interaction Libraries.
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.jmb.2020.03.011 Ryan M Woloschuk 1 , P Maximilian M Reed 1 , Sherin McDonald 2 , Maruti Uppalapati 2 , G Andrew Woolley 1
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.jmb.2020.03.011 Ryan M Woloschuk 1 , P Maximilian M Reed 1 , Sherin McDonald 2 , Maruti Uppalapati 2 , G Andrew Woolley 1
Affiliation
|
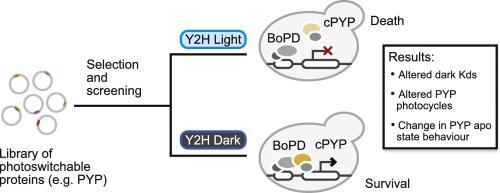
Although widely used in the detection and characterization of protein-protein interactions, Y2H screening has been under-used for the engineering of new optogenetic tools or the improvement of existing tools. Here we explore the feasibility of using Y2H selection and screening to evaluate libraries of photoswitchable protein-protein interactions. We targeted the interaction between circularly permuted photoactive yellow protein (cPYP) and its binding partner binder of PYP dark-state (BoPD) by mutating a set of four surface residues of cPYP that contribute to the binding interface. A library of ~10,000 variants was expressed in yeast together with BoPD in a Y2H format. An initial selection for the cPYP/BoPD interaction was performed using a range of concentrations of the cPYP chromophore. As expected, the majority (>90% of cPYP variants) no longer bound to BoPD. Replica plating was then used to evaluate the photoswitchability of the surviving clones. Photoswitchable cPYP variants with BoPD affinities equal to, or higher than, native cPYP were recovered in addition to variants with altered photocycles and binders that interacted with BoPD as apo-proteins. Y2H results reflected protein-protein interaction affinity, expression, photoswitchability, and chromophore uptake, and correlated well with results obtained both in vitro and in mammalian cells. Thus, by systematic variation of selection parameters, Y2H screens can be effectively used to generate new optogenetic tools for controlling protein-protein interactions for use in diverse settings.
中文翻译:

酵母两杂交筛选光开关蛋白-蛋白质相互作用文库。
尽管Y2H筛选已广泛用于蛋白质-蛋白质相互作用的检测和表征,但并未被广泛用于新的光遗传学工具的工程设计或现有工具的改进。在这里,我们探讨了使用Y2H选择和筛选来评估光开关蛋白相互作用的库的可行性。我们通过突变一组有助于结合界面的cPYP的四个表面残基来靶向圆形排列的光敏黄色蛋白(cPYP)及其PYP暗态(BoPD)的结合伴侣结合剂之间的相互作用。酵母和BoPD一起以Y2H格式在酵母中表达了约10,000个变体的文库。使用一系列浓度的cPYP生色团进行cPYP / BoPD相互作用的初始选择。不出所料,大多数(> 90%的cPYP变体)不再绑定到BoPD。然后使用复制平板评估存活的克隆的光开关性。BoPD亲和力等于或高于天然cPYP的可光转换cPYP变异体,除了具有与BoPD相互作用为载脂蛋白的改变了光循环和结合剂的变异体之外,还得到了回收。Y2H结果反映了蛋白质-蛋白质相互作用的亲和力,表达,光开关性和生色团摄取,并且与在体外和在哺乳动物细胞中获得的结果都很好地相关。因此,通过选择参数的系统变化,Y2H筛选可以有效地用于生成新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种环境。BoPD亲和力等于或高于天然cPYP的可光转换cPYP变异体,除了具有与BoPD相互作用为载脂蛋白的改变了光循环和结合剂的变异体之外,还得到了回收。Y2H结果反映了蛋白质-蛋白质相互作用的亲和力,表达,光开关性和生色团摄取,并且与在体外和在哺乳动物细胞中获得的结果都很好地相关。因此,通过选择参数的系统变化,可以有效地使用Y2H筛查产生新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。BoPD亲和力等于或高于天然cPYP的可光转换cPYP变异体,除了具有与BoPD相互作用为载脂蛋白的改变了光循环和结合剂的变异体之外,还得到了回收。Y2H结果反映了蛋白质-蛋白质相互作用的亲和力,表达,光开关性和生色团摄取,并且与在体外和在哺乳动物细胞中获得的结果都很好地相关。因此,通过选择参数的系统变化,可以有效地使用Y2H筛查产生新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。表达,光开关性和生色团吸收,并与在体外和在哺乳动物细胞中获得的结果良好相关。因此,通过选择参数的系统变化,Y2H筛查可有效地用于生成新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。表达,光开关性和生色团吸收,并与在体外和在哺乳动物细胞中获得的结果良好相关。因此,通过选择参数的系统变化,Y2H筛查可有效地用于生成新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。
更新日期:2020-03-18
中文翻译:

酵母两杂交筛选光开关蛋白-蛋白质相互作用文库。
尽管Y2H筛选已广泛用于蛋白质-蛋白质相互作用的检测和表征,但并未被广泛用于新的光遗传学工具的工程设计或现有工具的改进。在这里,我们探讨了使用Y2H选择和筛选来评估光开关蛋白相互作用的库的可行性。我们通过突变一组有助于结合界面的cPYP的四个表面残基来靶向圆形排列的光敏黄色蛋白(cPYP)及其PYP暗态(BoPD)的结合伴侣结合剂之间的相互作用。酵母和BoPD一起以Y2H格式在酵母中表达了约10,000个变体的文库。使用一系列浓度的cPYP生色团进行cPYP / BoPD相互作用的初始选择。不出所料,大多数(> 90%的cPYP变体)不再绑定到BoPD。然后使用复制平板评估存活的克隆的光开关性。BoPD亲和力等于或高于天然cPYP的可光转换cPYP变异体,除了具有与BoPD相互作用为载脂蛋白的改变了光循环和结合剂的变异体之外,还得到了回收。Y2H结果反映了蛋白质-蛋白质相互作用的亲和力,表达,光开关性和生色团摄取,并且与在体外和在哺乳动物细胞中获得的结果都很好地相关。因此,通过选择参数的系统变化,Y2H筛选可以有效地用于生成新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种环境。BoPD亲和力等于或高于天然cPYP的可光转换cPYP变异体,除了具有与BoPD相互作用为载脂蛋白的改变了光循环和结合剂的变异体之外,还得到了回收。Y2H结果反映了蛋白质-蛋白质相互作用的亲和力,表达,光开关性和生色团摄取,并且与在体外和在哺乳动物细胞中获得的结果都很好地相关。因此,通过选择参数的系统变化,可以有效地使用Y2H筛查产生新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。BoPD亲和力等于或高于天然cPYP的可光转换cPYP变异体,除了具有与BoPD相互作用为载脂蛋白的改变了光循环和结合剂的变异体之外,还得到了回收。Y2H结果反映了蛋白质-蛋白质相互作用的亲和力,表达,光开关性和生色团摄取,并且与在体外和在哺乳动物细胞中获得的结果都很好地相关。因此,通过选择参数的系统变化,可以有效地使用Y2H筛查产生新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。表达,光开关性和生色团吸收,并与在体外和在哺乳动物细胞中获得的结果良好相关。因此,通过选择参数的系统变化,Y2H筛查可有效地用于生成新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。表达,光开关性和生色团吸收,并与在体外和在哺乳动物细胞中获得的结果良好相关。因此,通过选择参数的系统变化,Y2H筛查可有效地用于生成新的光遗传学工具,以控制蛋白质-蛋白质相互作用,以用于各种场合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号