当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure, interactions and membrane topology of HIV gp41 ectodomain sequences.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.bbamem.2020.183274 Christopher Aisenbrey 1 , Burkhard Bechinger 2
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.bbamem.2020.183274 Christopher Aisenbrey 1 , Burkhard Bechinger 2
Affiliation
|
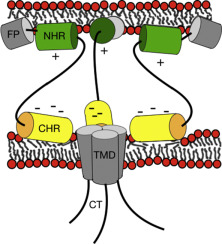
The gp41 type I membrane protein is part of the trimeric Env complex forming the spikes at the HIV surface. By interacting with cellular receptors, the Env protein complex initiates the infectious cycle of HIV. After the first contact has been established Env disassembles by shedding gp120 while the remaining gp41 undergoes a number of conformational changes which drive fusion of the cellular and the viral membranes. Here we investigated the membrane interactions and oligomerization of the two gp41 heptad repeat domains NHR and CHR. While these are thought to form a six-helix bundle in the post-fusion state little is known about their structure and role during prior fusion events. When investigated in aqueous buffer by CD and fluorescence quenching techniques the formation of NHR/CHR hetero-oligomers is detected. An equilibrium of monomers and hetero-oligomers is also observed in membrane environments. Furthermore, the partitioning to POPC or POPC/POPG 3/1 vesicles of the two domains alone or in combination has been studied. The membrane interactions were further characterized by 15N solid-state NMR spectroscopy of uniaxially oriented samples which shows that the polypeptide helices are oriented parallel to the bilayer surface. The 31P solid-state NMR spectra of the same samples are indicative of considerable disordering of the membrane packing. The data support models where NHR and CHR insert in the viral and cellular membranes, respectively, where they exhibit an active role in the membrane fusion events.
中文翻译:

HIV gp41胞外域序列的结构,相互作用和膜拓扑。
gp41 I型膜蛋白是三聚体Env复合体的一部分,在HIV表面形成尖峰。通过与细胞受体相互作用,Env蛋白复合物启动了HIV的感染周期。建立起第一次接触后,Env通过脱落gp120进行分解,而其余gp41经历了许多构象变化,这些构象变化驱动细胞膜和病毒膜融合。在这里,我们调查了两个gp41 heptad重复域NHR和CHR的膜相互作用和寡聚。尽管认为它们在融合后状态下形成六螺旋束,但对其在先前融合事件中的结构和作用知之甚少。当在水性缓冲液中通过CD和荧光猝灭技术进行研究时,检测到NHR / CHR杂聚物的形成。在膜环境中也观察到单体和杂聚物的平衡。此外,已经研究了单独地或组合地将两个结构域划分为POPC或POPC / POPG 3/1囊泡。通过单轴取向样品的15 N固态NMR光谱进一步表征了膜的相互作用,这表明多肽螺旋平行于双层表面取向。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。已经研究了单独或组合将两个结构域分配到POPC或POPC / POPG 3/1囊泡。通过单轴取向样品的15 N固态NMR光谱进一步表征了膜的相互作用,这表明多肽螺旋平行于双层表面取向。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。已经研究了单独或组合将两个结构域分配到POPC或POPC / POPG 3/1囊泡。通过单轴取向样品的15 N固态NMR光谱进一步表征了膜的相互作用,这表明多肽螺旋平行于双层表面取向。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。
更新日期:2020-03-19
中文翻译:

HIV gp41胞外域序列的结构,相互作用和膜拓扑。
gp41 I型膜蛋白是三聚体Env复合体的一部分,在HIV表面形成尖峰。通过与细胞受体相互作用,Env蛋白复合物启动了HIV的感染周期。建立起第一次接触后,Env通过脱落gp120进行分解,而其余gp41经历了许多构象变化,这些构象变化驱动细胞膜和病毒膜融合。在这里,我们调查了两个gp41 heptad重复域NHR和CHR的膜相互作用和寡聚。尽管认为它们在融合后状态下形成六螺旋束,但对其在先前融合事件中的结构和作用知之甚少。当在水性缓冲液中通过CD和荧光猝灭技术进行研究时,检测到NHR / CHR杂聚物的形成。在膜环境中也观察到单体和杂聚物的平衡。此外,已经研究了单独地或组合地将两个结构域划分为POPC或POPC / POPG 3/1囊泡。通过单轴取向样品的15 N固态NMR光谱进一步表征了膜的相互作用,这表明多肽螺旋平行于双层表面取向。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。已经研究了单独或组合将两个结构域分配到POPC或POPC / POPG 3/1囊泡。通过单轴取向样品的15 N固态NMR光谱进一步表征了膜的相互作用,这表明多肽螺旋平行于双层表面取向。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。已经研究了单独或组合将两个结构域分配到POPC或POPC / POPG 3/1囊泡。通过单轴取向样品的15 N固态NMR光谱进一步表征了膜的相互作用,这表明多肽螺旋平行于双层表面取向。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。相同样品的31P固态NMR光谱表明膜堆积严重紊乱。数据支持NHR和CHR分别插入病毒和细胞膜的模型,它们在膜融合事件中发挥积极作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号