Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Asymmetric Hydrosilylation of Vinylcyclopropanes via Stereospecific C-C Bond Cleavage.
iScience ( IF 4.6 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.isci.2020.100985 Chenhui Chen 1 , Hongliang Wang 1 , Yufeng Sun 1 , Jiayan Cui 1 , Jianbo Xie 1 , Yang Shi 1 , Shijia Yu 1 , Xin Hong 1 , Zhan Lu 1
中文翻译:

通过立体特异性CC键裂解,铁催化乙烯基环丙烷的不对称氢化硅烷化。
更新日期:2020-03-17
iScience ( IF 4.6 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.isci.2020.100985 Chenhui Chen 1 , Hongliang Wang 1 , Yufeng Sun 1 , Jiayan Cui 1 , Jianbo Xie 1 , Yang Shi 1 , Shijia Yu 1 , Xin Hong 1 , Zhan Lu 1
Affiliation
|
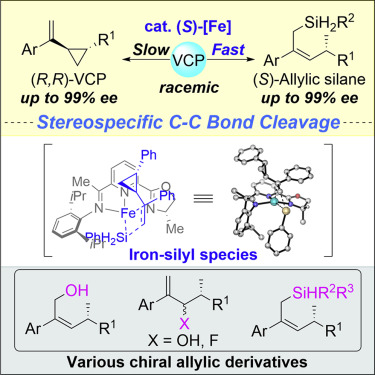
An iron-catalyzed highly anti-Markovnikov selective, enantioselective hydrosilylation of vinylcyclopropanes with PhSiH3 was reported for the preparation of valuable chiral allylic silanes via stereospecific C-C bond cleavage. Simultaneously, difficultly prepared chiral VCPs could be also obtained with moderate to excellent enantioselectivity via this kinetic resolution pathway. The chiral Z-allylic silanes could be converted to various chiral allylic derivatives. A possible mechanism via an iron-silyl species was proposed based on experimental and computational studies.
中文翻译:

通过立体特异性CC键裂解,铁催化乙烯基环丙烷的不对称氢化硅烷化。
据报道,铁的高度抗马尔可夫尼科夫乙烯基环丙烷与PhSiH 3的对映选择性氢化硅烷化反应可通过立体定向CC键裂解制备有价值的手性烯丙基硅烷。同时,也可以通过该动力学拆分途径以中等至优异的对映选择性获得难以制备的手性VCP。手性Z-烯丙基硅烷可以转化为各种手性烯丙基衍生物。在实验和计算研究的基础上,提出了一种可能的机制是通过甲硅烷基铁。











































 京公网安备 11010802027423号
京公网安备 11010802027423号