Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Drosophila OTK Is a Glycosaminoglycan-Binding Protein with High Conformational Flexibility.
Structure ( IF 4.4 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.str.2020.02.008 Daniel Rozbesky 1 , Jim Monistrol 1 , Vitul Jain 1 , James Hillier 1 , Sergi Padilla-Parra 2 , E Yvonne Jones 1
Structure ( IF 4.4 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.str.2020.02.008 Daniel Rozbesky 1 , Jim Monistrol 1 , Vitul Jain 1 , James Hillier 1 , Sergi Padilla-Parra 2 , E Yvonne Jones 1
Affiliation
|
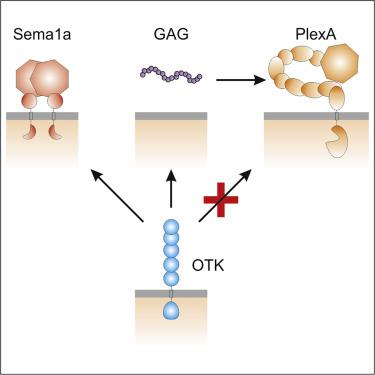
The transmembrane protein OTK plays an essential role in plexin and Wnt signaling during Drosophila development. We have determined a crystal structure of the last three domains of the OTK ectodomain and found that OTK shows high conformational flexibility resulting from mobility at the interdomain interfaces. We failed to detect direct binding between Drosophila Plexin A (PlexA) and OTK, which was suggested previously. We found that, instead of PlexA, OTK directly binds semaphorin 1a. Our binding analyses further revealed that glycosaminoglycans, heparin and heparan sulfate, are ligands for OTK and thus may play a role in the Sema1a-PlexA axon guidance system.
中文翻译:

果蝇 OTK 是一种具有高度构象灵活性的糖胺聚糖结合蛋白。
跨膜蛋白 OTK 在果蝇发育过程中的丛蛋白和 Wnt 信号传导中发挥重要作用。我们确定了 OTK 胞外域最后三个域的晶体结构,并发现 OTK 由于域间界面的迁移性而表现出高构象灵活性。我们未能检测到果蝇 Plexin A (PlexA) 和 OTK 之间的直接结合,正如之前所建议的那样。我们发现,OTK 直接结合信号蛋白 1a,而不是 PlexA。我们的结合分析进一步表明,糖胺聚糖、肝素和硫酸乙酰肝素是 OTK 的配体,因此可能在 Sema1a-PlexA 轴突引导系统中发挥作用。
更新日期:2020-03-17
中文翻译:

果蝇 OTK 是一种具有高度构象灵活性的糖胺聚糖结合蛋白。
跨膜蛋白 OTK 在果蝇发育过程中的丛蛋白和 Wnt 信号传导中发挥重要作用。我们确定了 OTK 胞外域最后三个域的晶体结构,并发现 OTK 由于域间界面的迁移性而表现出高构象灵活性。我们未能检测到果蝇 Plexin A (PlexA) 和 OTK 之间的直接结合,正如之前所建议的那样。我们发现,OTK 直接结合信号蛋白 1a,而不是 PlexA。我们的结合分析进一步表明,糖胺聚糖、肝素和硫酸乙酰肝素是 OTK 的配体,因此可能在 Sema1a-PlexA 轴突引导系统中发挥作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号