Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diapedesis-Induced Integrin Signaling via LFA-1 Facilitates Tissue Immunity by Inducing Intrinsic Complement C3 Expression in Immune Cells.
Immunity ( IF 25.5 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.immuni.2020.02.006 Martin Kolev 1 , Erin E West 1 , Natalia Kunz 1 , Daniel Chauss 2 , E Ashley Moseman 3 , Jubayer Rahman 3 , Tilo Freiwald 2 , Maria L Balmer 4 , Jonas Lötscher 4 , Sarah Dimeloe 5 , Elizabeth C Rosser 6 , Lucy R Wedderburn 7 , Katrin D Mayer-Barber 8 , Andrea Bohrer 8 , Paul Lavender 9 , Andrew Cope 9 , Luopin Wang 10 , Mariana J Kaplan 11 , Niki M Moutsopoulos 12 , Dorian McGavern 3 , Steven M Holland 13 , Christoph Hess 14 , Majid Kazemian 10 , Behdad Afzali 2 , Claudia Kemper 15
Immunity ( IF 25.5 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.immuni.2020.02.006 Martin Kolev 1 , Erin E West 1 , Natalia Kunz 1 , Daniel Chauss 2 , E Ashley Moseman 3 , Jubayer Rahman 3 , Tilo Freiwald 2 , Maria L Balmer 4 , Jonas Lötscher 4 , Sarah Dimeloe 5 , Elizabeth C Rosser 6 , Lucy R Wedderburn 7 , Katrin D Mayer-Barber 8 , Andrea Bohrer 8 , Paul Lavender 9 , Andrew Cope 9 , Luopin Wang 10 , Mariana J Kaplan 11 , Niki M Moutsopoulos 12 , Dorian McGavern 3 , Steven M Holland 13 , Christoph Hess 14 , Majid Kazemian 10 , Behdad Afzali 2 , Claudia Kemper 15
Affiliation
|
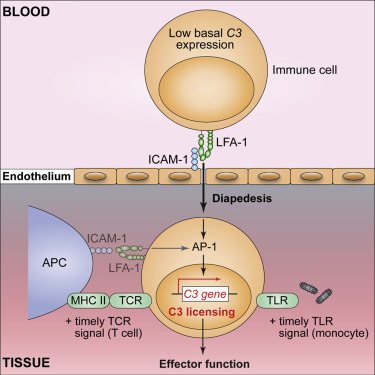
Intrinsic complement C3 activity is integral to human T helper type 1 (Th1) and cytotoxic T cell responses. Increased or decreased intracellular C3 results in autoimmunity and infections, respectively. The mechanisms regulating intracellular C3 expression remain undefined. We identified complement, including C3, as among the most significantly enriched biological pathway in tissue-occupying cells. We generated C3-reporter mice and confirmed that C3 expression was a defining feature of tissue-immune cells, including T cells and monocytes, occurred during transendothelial diapedesis, and depended on integrin lymphocyte-function-associated antigen 1 (LFA-1) signals. Immune cells from patients with leukocyte adhesion deficiency type 1 (LAD-1) had reduced C3 transcripts and diminished effector activities, which could be rescued proportionally by intracellular C3 provision. Conversely, increased C3 expression by T cells from arthritis patients correlated with disease severity. Our study defines integrins as key controllers of intracellular complement, demonstrates that perturbations in the LFA-1-C3-axis contribute to primary immunodeficiency, and identifies intracellular C3 as biomarker of severity in autoimmunity.
中文翻译:

经 LFA-1 的 Diapedesis 诱导的整合素信号传导通过诱导免疫细胞中的内在补体 C3 表达来促进组织免疫。
内在补体 C3 活性是人类 T 辅助细胞 1 型 (Th1) 和细胞毒性 T 细胞反应的组成部分。细胞内 C3 的增加或减少分别导致自身免疫和感染。调节细胞内 C3 表达的机制仍未确定。我们确定了补体,包括 C3,是组织占据细胞中最显着富集的生物途径之一。我们生成了 C3 报告基因小鼠,并证实 C3 表达是组织免疫细胞(包括 T 细胞和单核细胞)的一个决定性特征,发生在经内皮细胞渗出过程中,并且依赖于整合素淋巴细胞功能相关抗原 1 (LFA-1) 信号。来自 1 型白细胞粘附缺陷 (LAD-1) 患者的免疫细胞 C3 转录物减少,效应子活性降低,这可以通过细胞内 C3 供应按比例挽救。相反,关节炎患者的 T 细胞增加的 C3 表达与疾病严重程度相关。我们的研究将整合素定义为细胞内补体的关键控制器,证明 LFA-1-C3 轴的扰动有助于原发性免疫缺陷,并将细胞内 C3 确定为自身免疫严重程度的生物标志物。
更新日期:2020-03-19
中文翻译:

经 LFA-1 的 Diapedesis 诱导的整合素信号传导通过诱导免疫细胞中的内在补体 C3 表达来促进组织免疫。
内在补体 C3 活性是人类 T 辅助细胞 1 型 (Th1) 和细胞毒性 T 细胞反应的组成部分。细胞内 C3 的增加或减少分别导致自身免疫和感染。调节细胞内 C3 表达的机制仍未确定。我们确定了补体,包括 C3,是组织占据细胞中最显着富集的生物途径之一。我们生成了 C3 报告基因小鼠,并证实 C3 表达是组织免疫细胞(包括 T 细胞和单核细胞)的一个决定性特征,发生在经内皮细胞渗出过程中,并且依赖于整合素淋巴细胞功能相关抗原 1 (LFA-1) 信号。来自 1 型白细胞粘附缺陷 (LAD-1) 患者的免疫细胞 C3 转录物减少,效应子活性降低,这可以通过细胞内 C3 供应按比例挽救。相反,关节炎患者的 T 细胞增加的 C3 表达与疾病严重程度相关。我们的研究将整合素定义为细胞内补体的关键控制器,证明 LFA-1-C3 轴的扰动有助于原发性免疫缺陷,并将细胞内 C3 确定为自身免疫严重程度的生物标志物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号