Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.bmc.2020.115443 Amra Ibric , Verena Battisti , Sophie Deckardt , Anna Veronika Haller , Calvin Lee , Corinna Prötsch , Thierry Langer , Petra Heffeter , Hemma Henrike Schueffl , Brigitte Marian , Norbert Haider
|
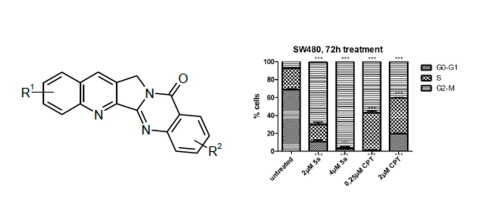
A series of new Luotonin A derivatives with substituents at rings A and E was synthesized, together with some E-ring-unsubstituted derivatives. Subsequently, the compound library was examined in silico for their binding into a previously proposed site in the DNA/topoisomerase I binary complex. Whereas no convincing correlation between docking scores and biological data from in vitro assays could be found, one novel 4,9-diamino Luotonin A derivative had strong antiproliferative activity based on massive G2/M phase arrest. As this biological activity clearly differs from the reference compound Camptothecin, this strongly indicates that at least some Luotonin A derivatives may be potent antiproliferative agents, however with a different mode of action.
中文翻译:

细胞毒性生物碱褪黑素A环和E环的修饰:合成,计算和生物学研究
合成了一系列在环A和E上具有取代基的新的褪黑素A衍生物,以及一些未被E环取代的衍生物。随后,对该化合物文库进行计算机分析,以检查其与DNA /拓扑异构酶I二元复合物中先前提议的位点的结合。尽管没有发现对接分数与来自体外测定的生物学数据之间令人信服的相关性,但一种新型的4,9-二氨基荧光素A衍生物基于大量的G2 / M期阻滞而具有很强的抗增殖活性。由于这种生物学活性明显不同于参考化合物喜树碱,因此有力地表明至少某些褪黑素A衍生物可能是有效的抗增殖剂,但作用方式不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号