Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.tetlet.2020.151850 Kundan Shaw , Sovan Niyogi , Vishnumaya Bisai
|
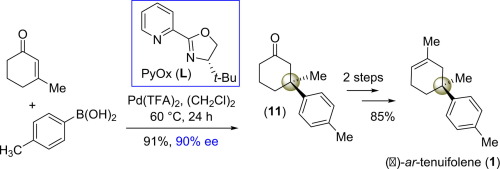
First catalytic asymmetric total synthesis of aromatic sesquiterpene, (−)-ar-teunifolene (1) is featured (3 steps, 75% overall yields) from commercially available 3-methyl cyclohex-2-enone 16. The enantioenriched 3,3-disubstituted cyclohexanone 11 is obtained from Pd(II)-catalyzed enantioselective (p-tolyl)boronic acid addition to 3-methyl cyclohex-2-enone 16 in 90% ee, which is found to be the key intermediate. A diastereoselective methyllithium addition of this enantioenriched product followed by dehydration completes straightforward access to (−)-ar-teunifolene (1).
中文翻译:

( - )- ar -Tenuifolene的催化对映选择性全合成
从商业上可买到的3-甲基环己-2-烯酮16(3个步骤,总产率75%)进行芳香族倍半萜(-)- ar- teunifolene(1)的首次催化不对称全合成。富含对映体的3,3-二取代的环己酮11是由Pd(II)催化的对映选择性(对甲苯基)硼酸在90%ee中加到3-甲基环己-2-烯酮16中获得的,这是关键的中间体。对该对映体富集的产物进行非对映选择性甲基锂加成后再进行脱水即可直接获得(-)- ar- teunifolene(1)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号