Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.apcatb.2020.118888 Yanfang Song , Yonghui Zhao , Guizhen Nan , Wei Chen , Zhikai Guo , Shenggang Li , Zhiyong Tang , Wei Wei , Yuhan Sun
|
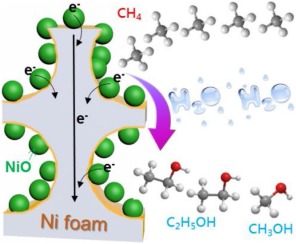
Electrocatalytic conversion of methane (CH4) to valuable chemicals under mild conditions is an attractive approach that combines the direct utilization of natural gas as a hydrocarbon feedstock and the chemical storage of renewable electricity. However, it remains a great challenge due to the intrinsic chemical inertness of CH4. Here we report that a NiO/Ni interface constructed by calcination can act as the active site for the electrooxidation of CH4 to alcohols especially ethanol. With the optimized NiO/Ni interface catalyst, an 89% Faradaic efficiency (FE) for ethanol production with a yield of 25 μmol∙gNiO-1∙h-1 at 1.40 V versus reversible hydrogen electrode (RHE) was achieved. Experiments and density functional theory (DFT) calculations demonstrated that the NiO/Ni interface enables efficient C–H activation and C–C coupling, leading to the highly selective formation of ethanol from CH4 electrooxidation.
中文翻译:

通过NiO / Ni界面将甲烷电催化氧化为乙醇
在温和的条件下将甲烷(CH 4)电催化转化为有价值的化学品是一种有吸引力的方法,该方法将直接利用天然气作为碳氢化合物原料和化学储存可再生电力相结合。然而,由于CH 4固有的化学惰性,它仍然是一个巨大的挑战。在这里,我们报道通过煅烧构造的NiO / Ni界面可以充当CH 4氧化成醇(尤其是乙醇)的活性位。通过优化的NiO / Ni界面催化剂,乙醇生产的法拉第效率(FE)为89%,产量为25μmol∙g NiO -1 ∙h -1相对于可逆氢电极(RHE),在1.40 V时达到了。实验和密度泛函理论(DFT)计算表明,NiO / Ni界面可实现有效的C–H活化和C–C偶联,从而导致CH 4电氧化过程中乙醇的高度选择性形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号