当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering a Self-Assembling Leucine Zipper Hydrogel System with Function-Specific Motifs for Tissue Regeneration
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-03-17 , DOI: 10.1021/acsbiomaterials.0c00026 Chun-Chieh Huang 1 , Sriram Ravindran 1 , Miya Kang 1 , Lyndon F. Cooper 1 , Anne George 1
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-03-17 , DOI: 10.1021/acsbiomaterials.0c00026 Chun-Chieh Huang 1 , Sriram Ravindran 1 , Miya Kang 1 , Lyndon F. Cooper 1 , Anne George 1
Affiliation
|
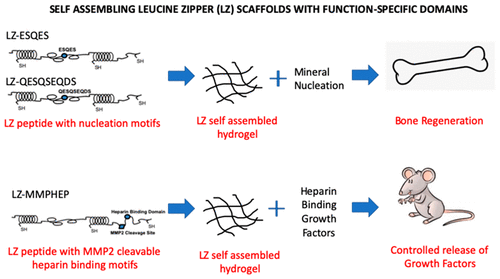
Protein-based self-assembling hydrogels can exhibit remarkably tunable properties as a scaffold for regenerative medicine applications. In this study, we sought to develop a leucine zipper (LZ) based self-assembling hydrogel with function-specific motifs for tissue-specific regeneration. As a proof-of-concept approach, we incorporated (a) calcium-binding domains ESQES and QESQSEQS derived from dentin matrix protein 1 (DMP1) and (b) an heparin-binding domain adjacent preceded by an MMP2 (matrix metalloprotease 2) cleavage site to facilitate loading of heparin binding growth factors, such as BMP-2, VEGF, and TGF-β1, and their release in vivo by endogenous MMP2 proteolytic cleavage. These scaffolds were characterized and evaluated in vitro and in vivo. In vivo studies highlighted the potential of the engineered LZ hydrogel with respect to osteogenic differentiation of stem cells. The premineralized LZ scaffold loaded with HMSCs showed an enhanced osteoinductive property when compared with the control nonmineralized scaffold. The LZ backbone with heparin-binding domain containing an MMP2 cleavage site facilitated tethering of heparin-binding growth factors, such as VEGF, TGF-β1 and BMP2 and demonstrated controlled release of these active growth factor both in vitro and in vivo and demonstrated growth factor specific activity in vivo (BMP-2 and TGF-β1). Overall, we present a versatile protein based self-assembling system with tunable properties for tissue regeneration.
中文翻译:

设计具有特定功能的自组装亮氨酸拉链水凝胶系统,用于组织再生。
基于蛋白质的自组装水凝胶可作为再生医学应用的支架展现出显着的可调性。在这项研究中,我们试图开发一种基于亮氨酸拉链(LZ)的自组装水凝胶,该凝胶具有功能特异性的图案,用于组织特异性的再生。作为概念验证的方法,我们并入了(a)源自牙本质基质蛋白1(DMP1)的钙结合结构域ESQES和QESQSEQS,以及(b)相邻的肝素结合结构域,随后是MMP2(基质金属蛋白酶2)裂解。该位点促进肝素结合生长因子(例如BMP-2,VEGF和TGF-β1)的加载,并通过内源性MMP2蛋白水解切割在体内释放。在体外和体内对这些支架进行了表征和评估。体内研究突出了工程化LZ水凝胶在干细胞成骨分化方面的潜力。与对照非矿化支架相比,装载有HMSC的预矿化LZ支架显示出增强的骨诱导特性。具有包含MMP2裂解位点的肝素结合结构域的LZ骨架促进了肝素结合生长因子(如VEGF,TGF-β1和BMP2)的束缚,并在体外和体内证明了这些活性生长因子的受控释放,并证明了生长因子体内的特定活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。与对照非矿化支架相比,装载有HMSC的预矿化LZ支架显示出增强的骨诱导特性。具有包含MMP2裂解位点的肝素结合结构域的LZ骨架促进了肝素结合生长因子(如VEGF,TGF-β1和BMP2)的束缚,并在体外和体内证明了这些活性生长因子的受控释放,并证明了生长因子体内的特定活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。与对照非矿化支架相比,装载有HMSC的预矿化LZ支架显示出增强的骨诱导特性。具有包含MMP2切割位点的肝素结合域的LZ骨架促进了肝素结合生长因子(例如VEGF,TGF-β1和BMP2)的束缚,并在体外和体内证明了这些活性生长因子的受控释放,并证明了生长因子体内的特定活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。TGF-β1和BMP2并在体内外证明了这些活性生长因子的受控释放,并在体内证明了生长因子的比活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。TGF-β1和BMP2并在体内外证明了这些活性生长因子的受控释放,并在体内证明了生长因子的比活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。
更新日期:2020-03-17
中文翻译:

设计具有特定功能的自组装亮氨酸拉链水凝胶系统,用于组织再生。
基于蛋白质的自组装水凝胶可作为再生医学应用的支架展现出显着的可调性。在这项研究中,我们试图开发一种基于亮氨酸拉链(LZ)的自组装水凝胶,该凝胶具有功能特异性的图案,用于组织特异性的再生。作为概念验证的方法,我们并入了(a)源自牙本质基质蛋白1(DMP1)的钙结合结构域ESQES和QESQSEQS,以及(b)相邻的肝素结合结构域,随后是MMP2(基质金属蛋白酶2)裂解。该位点促进肝素结合生长因子(例如BMP-2,VEGF和TGF-β1)的加载,并通过内源性MMP2蛋白水解切割在体内释放。在体外和体内对这些支架进行了表征和评估。体内研究突出了工程化LZ水凝胶在干细胞成骨分化方面的潜力。与对照非矿化支架相比,装载有HMSC的预矿化LZ支架显示出增强的骨诱导特性。具有包含MMP2裂解位点的肝素结合结构域的LZ骨架促进了肝素结合生长因子(如VEGF,TGF-β1和BMP2)的束缚,并在体外和体内证明了这些活性生长因子的受控释放,并证明了生长因子体内的特定活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。与对照非矿化支架相比,装载有HMSC的预矿化LZ支架显示出增强的骨诱导特性。具有包含MMP2裂解位点的肝素结合结构域的LZ骨架促进了肝素结合生长因子(如VEGF,TGF-β1和BMP2)的束缚,并在体外和体内证明了这些活性生长因子的受控释放,并证明了生长因子体内的特定活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。与对照非矿化支架相比,装载有HMSC的预矿化LZ支架显示出增强的骨诱导特性。具有包含MMP2切割位点的肝素结合域的LZ骨架促进了肝素结合生长因子(例如VEGF,TGF-β1和BMP2)的束缚,并在体外和体内证明了这些活性生长因子的受控释放,并证明了生长因子体内的特定活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。TGF-β1和BMP2并在体内外证明了这些活性生长因子的受控释放,并在体内证明了生长因子的比活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。TGF-β1和BMP2并在体内外证明了这些活性生长因子的受控释放,并在体内证明了生长因子的比活性(BMP-2和TGF-β1)。总体而言,我们提出了一种基于通用蛋白质的自组装系统,该系统具有可调节的组织再生特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号