当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ginsenoside Rg2 Ameliorates High-Fat Diet-Induced Metabolic Disease through SIRT1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jafc.0c00833 Bo Cheng 1, 2 , Weihang Gao 1 , Xiaojie Wu 3 , Mingxuan Zheng 4 , Yuanyuan Yu 5 , Chunhui Song 1 , Wei Miao 1 , Zehong Yang 6 , Yuqing He 1 , Changhui Liu 7 , Wangyin Yang 8 , Xiaoying Yang 4 , Yanwu Li 1 , Fang Zhang 3 , Yong Gao 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jafc.0c00833 Bo Cheng 1, 2 , Weihang Gao 1 , Xiaojie Wu 3 , Mingxuan Zheng 4 , Yuanyuan Yu 5 , Chunhui Song 1 , Wei Miao 1 , Zehong Yang 6 , Yuqing He 1 , Changhui Liu 7 , Wangyin Yang 8 , Xiaoying Yang 4 , Yanwu Li 1 , Fang Zhang 3 , Yong Gao 1, 2
Affiliation
|
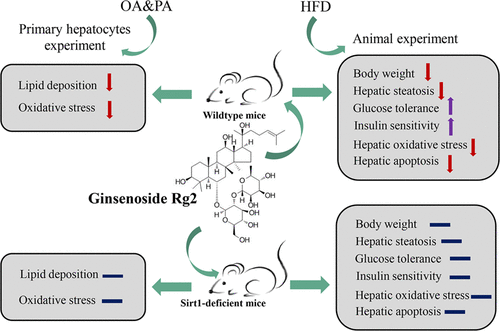
Ginsenoside Rg2 has been previously reported to reduce glucose production and adipogenesis in adipose tissue. However, the effects of ginsenosides Rg2 on hepatic lipid metabolism remain vacant. In this study, we found that ginsenoside Rg2 treatment significantly attenuated oleic acid and palmitic acid (OA&PA)-induced intracellular lipid deposition and oxidative stress in mouse primary hepatocytes. C57BL/6J mice that are fed with a high-fat diet (HFD) and treated with ginsenosides Rg2 displayed decreased body weight, reversed hepatic steatosis, and improved glucose tolerance and insulin sensitivity. Ginsenoside Rg2 administration significantly ameliorated HFD-induced hepatic oxidative stress and apoptosis. Moreover, Ginsenoside Rg2 had a good affinity with Sirtuin1 (SIRT1) and regulated its expression in vivo and in vitro. Deficiency of SIRT1 eliminated the therapeutic effect of ginsenoside Rg2 on lipid accumulation and overproduction of reactive oxygen species (ROS) in OA&PA-induced mice primary hepatocytes. Ginsenoside Rg2 treatment failed to alter the lipid and glucose disorder in hepatic SIRT1 deficient mice feeding on HFD. SIRT1 deficiency dissolves the therapeutic effect of ginsenoside Rg2 on oxidative stress and hepatocyte apoptosis induced by HFD. In summary, ginsenoside Rg2 plays a therapeutic role in HFD-induced hepatosteatosis of mice by decreasing the lipogenesis process and improving antioxidant capacity in an SIRT1-dependent manner.
中文翻译:

人参皂甙Rg2通过SIRT1改善高脂饮食诱导的代谢性疾病
人参皂苷Rg2先前已报道可减少脂肪组织中的葡萄糖生成和脂肪生成。然而,人参皂甙Rg2对肝脂质代谢的作用仍然是空缺的。在这项研究中,我们发现人参皂苷Rg2处理显着减弱了小鼠原代肝细胞中的油酸和棕榈酸(OA&PA)诱导的细胞内脂质沉积和氧化应激。饲喂高脂饮食(HFD)并用人参皂甙Rg2治疗的C57BL / 6J小鼠,体重减轻,肝脂肪变性逆转,并且葡萄糖耐量和胰岛素敏感性提高。人参皂甙Rg2的使用可显着改善HFD诱导的肝氧化应激和细胞凋亡。此外,人参皂苷Rg2与Sirtuin1(SIRT1)具有良好的亲和力,并在体内和体外调节其表达。SIRT1的缺乏消除了人参皂苷Rg2对OA&PA诱导的小鼠原代肝细胞脂质积累和活性氧(ROS)过量产生的治疗作用。人参皂甙Rg2处理未能改变以HFD为食的肝SIRT1缺陷小鼠的脂质和葡萄糖异常。SIRT1缺乏症消除了人参皂苷Rg2对HFD诱导的氧化应激和肝细胞凋亡的治疗作用。总之,人参皂苷Rg2通过减少脂肪形成过程并以SIRT1依赖性方式提高抗氧化能力,在HFD诱导的小鼠肝脂肪变性中起治疗作用。人参皂甙Rg2处理未能改变以HFD为食的肝SIRT1缺陷小鼠的脂质和葡萄糖异常。SIRT1缺乏症消除了人参皂苷Rg2对HFD诱导的氧化应激和肝细胞凋亡的治疗作用。总之,人参皂苷Rg2通过减少脂肪形成过程并以SIRT1依赖性方式提高抗氧化能力,在HFD诱导的小鼠肝脂肪变性中起治疗作用。人参皂甙Rg2处理未能改变以HFD为食的肝SIRT1缺陷小鼠的脂质和葡萄糖异常。SIRT1缺乏症消除了人参皂甙Rg2对HFD诱导的氧化应激和肝细胞凋亡的治疗作用。总之,人参皂苷Rg2通过减少脂肪形成过程并以SIRT1依赖性方式提高抗氧化能力,在HFD诱导的小鼠肝脂肪变性中起治疗作用。
更新日期:2020-03-27
中文翻译:

人参皂甙Rg2通过SIRT1改善高脂饮食诱导的代谢性疾病
人参皂苷Rg2先前已报道可减少脂肪组织中的葡萄糖生成和脂肪生成。然而,人参皂甙Rg2对肝脂质代谢的作用仍然是空缺的。在这项研究中,我们发现人参皂苷Rg2处理显着减弱了小鼠原代肝细胞中的油酸和棕榈酸(OA&PA)诱导的细胞内脂质沉积和氧化应激。饲喂高脂饮食(HFD)并用人参皂甙Rg2治疗的C57BL / 6J小鼠,体重减轻,肝脂肪变性逆转,并且葡萄糖耐量和胰岛素敏感性提高。人参皂甙Rg2的使用可显着改善HFD诱导的肝氧化应激和细胞凋亡。此外,人参皂苷Rg2与Sirtuin1(SIRT1)具有良好的亲和力,并在体内和体外调节其表达。SIRT1的缺乏消除了人参皂苷Rg2对OA&PA诱导的小鼠原代肝细胞脂质积累和活性氧(ROS)过量产生的治疗作用。人参皂甙Rg2处理未能改变以HFD为食的肝SIRT1缺陷小鼠的脂质和葡萄糖异常。SIRT1缺乏症消除了人参皂苷Rg2对HFD诱导的氧化应激和肝细胞凋亡的治疗作用。总之,人参皂苷Rg2通过减少脂肪形成过程并以SIRT1依赖性方式提高抗氧化能力,在HFD诱导的小鼠肝脂肪变性中起治疗作用。人参皂甙Rg2处理未能改变以HFD为食的肝SIRT1缺陷小鼠的脂质和葡萄糖异常。SIRT1缺乏症消除了人参皂苷Rg2对HFD诱导的氧化应激和肝细胞凋亡的治疗作用。总之,人参皂苷Rg2通过减少脂肪形成过程并以SIRT1依赖性方式提高抗氧化能力,在HFD诱导的小鼠肝脂肪变性中起治疗作用。人参皂甙Rg2处理未能改变以HFD为食的肝SIRT1缺陷小鼠的脂质和葡萄糖异常。SIRT1缺乏症消除了人参皂甙Rg2对HFD诱导的氧化应激和肝细胞凋亡的治疗作用。总之,人参皂苷Rg2通过减少脂肪形成过程并以SIRT1依赖性方式提高抗氧化能力,在HFD诱导的小鼠肝脂肪变性中起治疗作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号