当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intrinsic Chemical Reactivity of Silicon Electrode Materials: Gas Evolution
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-03-17 , DOI: 10.1021/acs.chemmater.0c00308 Claire L. Seitzinger 1 , Robert L. Sacci 1 , Jaclyn E. Coyle 2, 3 , Christopher A. Apblett 3 , Kevin A. Hays 1 , Ryan R. Armstrong 1 , Alexander M. Rogers 1 , Beth L. Armstrong 4 , Tyler H. Bennet 1 , Nathan R. Neale 2 , Gabriel M. Veith 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-03-17 , DOI: 10.1021/acs.chemmater.0c00308 Claire L. Seitzinger 1 , Robert L. Sacci 1 , Jaclyn E. Coyle 2, 3 , Christopher A. Apblett 3 , Kevin A. Hays 1 , Ryan R. Armstrong 1 , Alexander M. Rogers 1 , Beth L. Armstrong 4 , Tyler H. Bennet 1 , Nathan R. Neale 2 , Gabriel M. Veith 1
Affiliation
|
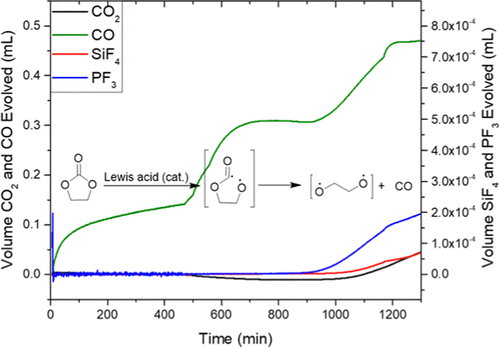
In this work, we explore how the chemical reactivity toward an aprotic battery electrolyte changes as a function of lithium salt and silicon surface termination chemistry. The reactions are highly correlated, where one decomposition reaction leads to a subsequent decomposition reaction. The data show that the presence of silicon hydrides (SiHx) promotes the formation of CO gas, while surface oxides SiOx drive the formation of CO2. The extent and rate of oxidation depend on the surface basicity of the SiO2 surface species. The most acidic surfaces seem to hinder CO2 generation but not the decomposition of the salt. Indeed, the presence of F-containing salts (LiPF6 and LiTFSI) promotes the reactions between carbonate electrolyte and silicon surfaces. Surfaces with high Li content seem to be the most passivating to gassing reactions, pointing to a pathway to stabilize the interfaces during cell formation and assembly.
中文翻译:

硅电极材料的内在化学反应性:气体逸出
在这项工作中,我们探索了对非质子电池电解质的化学反应性如何随锂盐和硅表面终止化学的变化而变化。这些反应是高度相关的,其中一个分解反应导致随后的分解反应。数据表明,氢化硅(SiH x)的存在促进了CO气体的形成,而表面氧化物SiO x推动了CO 2的形成。氧化的程度和速率取决于SiO 2表面物质的表面碱性。最酸性的表面似乎阻碍了CO 2的生成,但没有阻碍盐的分解。实际上,存在含F的盐(LiPF 6和LiTFSI)促进碳酸盐电解质和硅表面之间的反应。高Li含量的表面似乎对放气反应最钝化,表明在细胞形成和组装过程中稳定界面的途径。
更新日期:2020-04-23
中文翻译:

硅电极材料的内在化学反应性:气体逸出
在这项工作中,我们探索了对非质子电池电解质的化学反应性如何随锂盐和硅表面终止化学的变化而变化。这些反应是高度相关的,其中一个分解反应导致随后的分解反应。数据表明,氢化硅(SiH x)的存在促进了CO气体的形成,而表面氧化物SiO x推动了CO 2的形成。氧化的程度和速率取决于SiO 2表面物质的表面碱性。最酸性的表面似乎阻碍了CO 2的生成,但没有阻碍盐的分解。实际上,存在含F的盐(LiPF 6和LiTFSI)促进碳酸盐电解质和硅表面之间的反应。高Li含量的表面似乎对放气反应最钝化,表明在细胞形成和组装过程中稳定界面的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号