当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Investigation of the Vitamin K Epoxide Reductase (VKORC1) Binding Site with Vitamin K
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.biochem.9b01084 Nolan Chatron 1 , Rami Abi Khalil 1 , Etienne Benoit 1 , Virginie Lattard 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.biochem.9b01084 Nolan Chatron 1 , Rami Abi Khalil 1 , Etienne Benoit 1 , Virginie Lattard 1
Affiliation
|
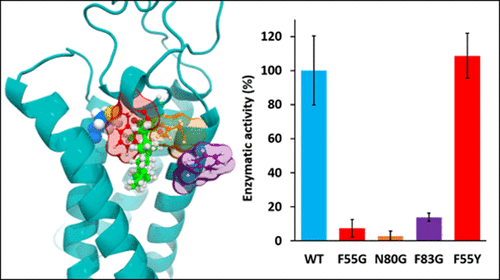
The vitamin K epoxide reductase (VKORC1) enzyme is of primary importance in many physiological processes, i.e., blood coagulation, energy metabolism, and arterial calcification prevention, due to its role in the vitamin K cycle. Indeed, VKORC1 catalyzes reduction of vitamin K epoxide to quinone and then to hydroquinone. However, the three-dimensional VKORC1 structure remains experimentally undetermined, because of the endoplasmic reticulum membrane location of this enzyme. Here we present a molecular modeling investigation of the VKORC1 enzymatic site structure and function, supported by in vitro enzymatic assays. Four VKORC1 mutants were designed in silico (F55G, F55Y, N80G, and F83G) based on a previous study that identified residues F55, N80, and F83 as being crucial for vitamin K epoxide binding. F55G, N80G, and F83G nonconservative mutants were all predicted to be inactive by molecular modeling analyses. However, the F55Y conservative mutant was expected to be active compared to wild-type VKORC1. In vitro enzymatic assays performed on recombinant proteins assessed our molecular modeling hypotheses and led us to describe the role of accurate VKORC1 active site residues with respect to VKORC1. Residues F55, N80, and F83 appeared to act in a concerted manner to keep vitamin K epoxide close to the C135 catalytic residue. Residues F55 and N80 prevent naphthoquinone head rotation away from the active site, assisted by residue F83 that prevents vitamin K from sliding outside the enzymatic pocket, through hydrophobic tail stabilization. Our results thus highlighted the specific functions of VKORC1 catalytic pocket residues and evidenced the ability of our structural model to predict biological effects of VKORC1 mutations.
中文翻译:

维生素K与维生素K结合位点(VKORC1)结合结构的结构研究
由于维生素K环氧还原酶(VKORC1)在维生素K循环中的作用,因此在许多生理过程中(血液凝固,能量代谢和预防动脉钙化)至关重要。实际上,VKORC1催化将维生素K环氧化物还原为醌,然后还原为对苯二酚。但是,由于该酶的内质网膜位置,三维VKORC1结构在实验上尚未确定。在这里,我们介绍了VKORC1酶学位点结构和功能的分子模型研究,并得到了体外酶学分析的支持。在计算机上设计了四个VKORC1突变体(F55G,F55Y,N80G和F83G)基于先前的研究,该研究确定残基F55,N80和F83对于维生素K环氧化物的结合至关重要。通过分子模型分析,F55G,N80G和F83G非保守突变体均被预测为无活性。但是,与野生型VKORC1相比,F55Y保守突变体有望发挥作用。体外对重组蛋白进行的酶促分析评估了我们的分子建模假设,使我们描述了准确的VKORC1活性位点残基相对于VKORC1的作用。残基F55,N80和F83似乎起着协调作用,以使维生素K环氧化物保持接近C135催化残基。残基F55和N80阻止了萘醌头旋转离开活性位点,而残基F83则通过疏水尾部稳定作用阻止了维生素K滑到酶口袋之外。因此,我们的结果突出了VKORC1催化口袋残基的特定功能,并证明了我们的结构模型预测VKORC1突变的生物学效应的能力。
更新日期:2020-03-24
中文翻译:

维生素K与维生素K结合位点(VKORC1)结合结构的结构研究
由于维生素K环氧还原酶(VKORC1)在维生素K循环中的作用,因此在许多生理过程中(血液凝固,能量代谢和预防动脉钙化)至关重要。实际上,VKORC1催化将维生素K环氧化物还原为醌,然后还原为对苯二酚。但是,由于该酶的内质网膜位置,三维VKORC1结构在实验上尚未确定。在这里,我们介绍了VKORC1酶学位点结构和功能的分子模型研究,并得到了体外酶学分析的支持。在计算机上设计了四个VKORC1突变体(F55G,F55Y,N80G和F83G)基于先前的研究,该研究确定残基F55,N80和F83对于维生素K环氧化物的结合至关重要。通过分子模型分析,F55G,N80G和F83G非保守突变体均被预测为无活性。但是,与野生型VKORC1相比,F55Y保守突变体有望发挥作用。体外对重组蛋白进行的酶促分析评估了我们的分子建模假设,使我们描述了准确的VKORC1活性位点残基相对于VKORC1的作用。残基F55,N80和F83似乎起着协调作用,以使维生素K环氧化物保持接近C135催化残基。残基F55和N80阻止了萘醌头旋转离开活性位点,而残基F83则通过疏水尾部稳定作用阻止了维生素K滑到酶口袋之外。因此,我们的结果突出了VKORC1催化口袋残基的特定功能,并证明了我们的结构模型预测VKORC1突变的生物学效应的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号