当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Establishing a Link Between the Dual Cycles in Methanol-to-Olefins Conversion on H-ZSM-5: Aromatization of Cycloalkenes
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.0c00838 Min Hu 1, 2 , Chao Wang 1 , Xiuzhi Gao 3 , Yueying Chu 1 , Guodong Qi 1 , Qiang Wang 1 , Guangtong Xu 3 , Jun Xu 1, 4 , Feng Deng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.0c00838 Min Hu 1, 2 , Chao Wang 1 , Xiuzhi Gao 3 , Yueying Chu 1 , Guodong Qi 1 , Qiang Wang 1 , Guangtong Xu 3 , Jun Xu 1, 4 , Feng Deng 1
Affiliation
|
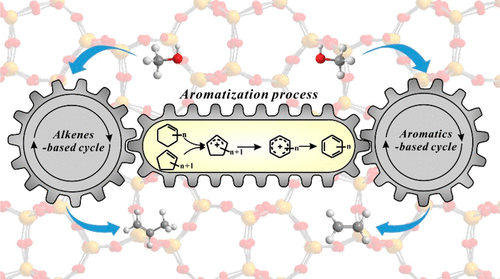
The aromatization of alkenes in the methanol-to-olefins (MTO) reaction on H-ZSM-5 zeolites was investigated by solid-state NMR and GC-MS spectroscopy. The formed cycloalkenes including cyclopentene and cyclohexene in the MTO reaction show high reactivity toward formation of aromatics. Cyclohexene tends to form cyclopentene through ring contraction, and the subsequent ring expansion leads to the production of aromatics. The ring-contraction/expansion process of cyclohexene is consistent with the 13C isotopic scrambling in aromatics. Solid-state NMR experiments evidenced the formation of cyclopentenyl cations and benzenium ions intermediates in the aromatization of C6 cycloalkenes. A plausible route to link the alkene-based and aromatic-based cycles in the MTO reaction on ZSM-5 zeolite was proposed, which is corroborated by DFT calculations.
中文翻译:

在H-ZSM-5上的甲醇制烯烃转化的双循环之间建立联系:环烯烃的芳构化
通过固态NMR和GC-MS光谱研究了H-ZSM-5沸石在甲醇制烯烃(MTO)反应中烯烃的芳构化作用。在MTO反应中形成的包括环戊烯和环己烯的环烯烃显示出对形成芳族化合物的高反应性。环己烯趋于通过环收缩形成环戊烯,随后的环膨胀导致芳族化合物的产生。环己烯的环缩/膨胀过程与13芳香族化合物中的C同位素加扰。固态NMR实验表明,在C6环烯烃的芳构化过程中,形成了环戊烯基阳离子和苯离子中间体。提出了在ZSM-5沸石上进行MTO反应时,将烯烃基和芳基基团连接起来的一条可行的途径,这通过DFT计算得到了证实。
更新日期:2020-03-21
中文翻译:

在H-ZSM-5上的甲醇制烯烃转化的双循环之间建立联系:环烯烃的芳构化
通过固态NMR和GC-MS光谱研究了H-ZSM-5沸石在甲醇制烯烃(MTO)反应中烯烃的芳构化作用。在MTO反应中形成的包括环戊烯和环己烯的环烯烃显示出对形成芳族化合物的高反应性。环己烯趋于通过环收缩形成环戊烯,随后的环膨胀导致芳族化合物的产生。环己烯的环缩/膨胀过程与13芳香族化合物中的C同位素加扰。固态NMR实验表明,在C6环烯烃的芳构化过程中,形成了环戊烯基阳离子和苯离子中间体。提出了在ZSM-5沸石上进行MTO反应时,将烯烃基和芳基基团连接起来的一条可行的途径,这通过DFT计算得到了证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号