当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Fischer–Tropsch Synthesis Rates by the Combined Presence of Aqueous and Organic Media in Biphasic Systems
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acscatal.9b04369 Felipe Anaya 1 , Daniel E. Resasco 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acscatal.9b04369 Felipe Anaya 1 , Daniel E. Resasco 1
Affiliation
|
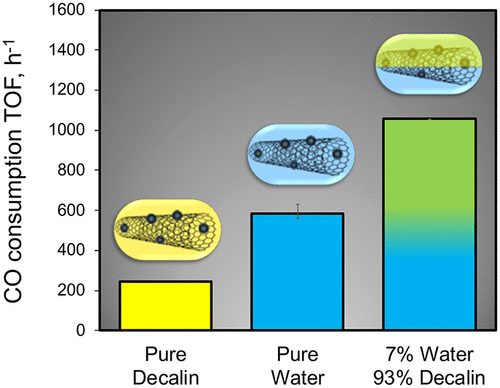
We report that when the Fischer–Tropsch synthesis (FTS) catalyzed by Ru particles supported on multiwall carbon nanotubes (Ru/CNT) is conducted in a biphasic decalin/water solvent mixture, the observed rate is significantly higher than in single-phase organic or aqueous medium. Multiwall carbon nanotubes of varying wettability and doped with Ru nanoparticles were tested as catalysts and stabilizers of biphasic emulsions in a batch reactor under FTS reaction conditions (H2:CO = 4:1, 220 °C, 55 bar). First, in comparison to the runs conducted in pure organic solvent, both rate and selectivity increased with the addition of a second (aqueous) phase. Likewise, a large increase in reaction rate was found when a second (organic) phase was incorporated, relative to that measured in pure aqueous phase. Notably, this rate increase was more substantial when the nanotubes employed were more hydrophobic and exhibited higher oil wettability. At the same time, for a given (Ru/CNT) catalyst, the rate increased with the oil/water solvent ratio, suggesting that not only the promotion by water but also the interaction of the catalyst surface with the organic solvents at the oil/water interfaces plays a key role in enabling higher rates. Because the rate changes are not due to changes in rates of mass transfer, it is concluded that the intrinsic kinetics was enhanced. Among the possible explanations for the enhanced kinetics, we discuss here some concepts recently proposed in the literature, including the water-promotion of H-assisted CO dissociation and the disruption of dense CO surface layers by the organic solvent.
中文翻译:

水和有机介质在双相系统中的结合提高了费托合成速率
我们报告说,当在多相碳氢萘/水溶剂混合物中以多壁碳纳米管(Ru / CNT)负载的Ru颗粒催化的费-托合成(FTS)时,观察到的速率明显高于单相有机或有机溶剂。水性介质。在FTS反应条件下(H 2),在间歇式反应器中测试了不同润湿性并掺有Ru纳米颗粒的多壁碳纳米管作为双相乳液的催化剂和稳定剂。:CO = 4:1,220°C,55 bar)。首先,与在纯有机溶剂中进行的实验相比,添加第二(水)相可提高速率和选择性。同样,相对于纯水相测得的第二(有机)相,反应速率大大提高。值得注意的是,当所用的纳米管疏水性更高并且表现出较高的油润湿性时,该速率增加更为显着。同时,对于给定的(Ru / CNT)催化剂,该速率随油/水溶剂比的增加而增加,这表明不仅水促进作用,而且催化剂表面与油/水溶剂之间的有机溶剂相互作用。水界面在提高水位方面起着关键作用。因为速率变化不是由于传质速率的变化引起的,结论是内在动力学得到增强。在增强动力学的可能解释中,我们在这里讨论文献中最近提出的一些概念,包括水促进H辅助CO的离解和致密的CO表面层被有机溶剂破坏。
更新日期:2020-03-27
中文翻译:

水和有机介质在双相系统中的结合提高了费托合成速率
我们报告说,当在多相碳氢萘/水溶剂混合物中以多壁碳纳米管(Ru / CNT)负载的Ru颗粒催化的费-托合成(FTS)时,观察到的速率明显高于单相有机或有机溶剂。水性介质。在FTS反应条件下(H 2),在间歇式反应器中测试了不同润湿性并掺有Ru纳米颗粒的多壁碳纳米管作为双相乳液的催化剂和稳定剂。:CO = 4:1,220°C,55 bar)。首先,与在纯有机溶剂中进行的实验相比,添加第二(水)相可提高速率和选择性。同样,相对于纯水相测得的第二(有机)相,反应速率大大提高。值得注意的是,当所用的纳米管疏水性更高并且表现出较高的油润湿性时,该速率增加更为显着。同时,对于给定的(Ru / CNT)催化剂,该速率随油/水溶剂比的增加而增加,这表明不仅水促进作用,而且催化剂表面与油/水溶剂之间的有机溶剂相互作用。水界面在提高水位方面起着关键作用。因为速率变化不是由于传质速率的变化引起的,结论是内在动力学得到增强。在增强动力学的可能解释中,我们在这里讨论文献中最近提出的一些概念,包括水促进H辅助CO的离解和致密的CO表面层被有机溶剂破坏。











































 京公网安备 11010802027423号
京公网安备 11010802027423号