当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrafast Heme Relaxation Dynamics Probing the Unfolded States of Cytochrome c Induced by Liposomes: Effect of Charge of Phospholipids
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jpcb.9b11957 Chinju Govind 1, 2 , Megha Paul 1, 2 , Venugopal Karunakaran 1, 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jpcb.9b11957 Chinju Govind 1, 2 , Megha Paul 1, 2 , Venugopal Karunakaran 1, 2
Affiliation
|
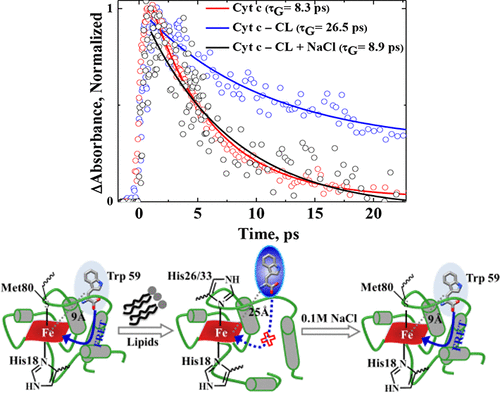
The ubiquitous electron transfer heme protein, Cytochrome c (Cyt c) catalyzes the peroxidation of cardiolipin (CL) in the early stage of apoptosis, where Cyt c undergoes conformational changes leading to the partial unfolding of the protein. Here the interaction dynamics of Cyt c with liposomes having different charges [CL, – 2; POPG (2-Oleoyl-1-palmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt), −1; and POPC (2-Oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine), 0] leading to various degrees of partial unfolding is investigated with steady state optical spectroscopy and femtosecond time-resolved pump–probe spectroscopy. The signature of the partial unfolding of the protein was observed in the absorption, fluorescence, and CD spectra of Cyt c–liposome complexes with an increase of lipid/protein (L/P) ratio, and the protein was refolded by the addition of 0.1 M of NaCl. The femtosecond transient absorption spectra of the complexes were measured by selectively exciting the heme and tryptophan (Trp) at 385 and 280 nm, respectively. Though significant changes were not observed in the excited state relaxation dynamics of the heme in liposomes by exciting at 385 nm, the 280 nm excitation exhibited a systematic increase of the excited state relaxation dynamics leading to the increase of lifetime of Trp and global conformational relaxation dynamics with the increase of anionic charge of the lipids. This reveals the decrease of efficiency of fluorescence resonance energy transfer from Trp to heme due to the increase of distance between them upon increase of partial unfolding of the proteins by liposomes. Such observation exhibits the Trp as a marker amino acid to reflect the dynamics of partial unfolding of the protein rising from the change in the tertiary structure and axial ligand interaction of the heme proteins in liposomes. The relaxation dynamics of the complexes in the presence of salt are similar to that of the protein alone, reflecting that the refolding of the protein and the interactions are dominated by electrostatic interaction rather than the hydrophobic interaction.
中文翻译:

超快血红素松弛动力学探测脂质体诱导的细胞色素c的未折叠状态:磷脂电荷的影响
普遍存在的电子转移血红素蛋白,细胞色素c(Cyt c)在细胞凋亡的早期催化心磷脂(CL)的过氧化,其中Cyt c发生构象变化,导致该蛋白的部分展开。Cyt c与具有不同电荷的脂质体的相互作用动力学[CL,– 2; POPG(2-油酰基-1-棕榈酰基-sn-甘油-3-磷酸-rac-(1-甘油)钠盐),-1; 和POPC(2-油酰基-1-棕榈酰基-sn-glycero-3-phosphocholine),0]用稳态光谱和飞秒时间分辨泵浦-探针光谱研究了导致不同程度的部分展开的部分。在Cyt c-脂质体复合物的吸收,荧光和CD光谱中观察到了蛋白质部分解开的特征,随着脂质/蛋白质(L / P)比的增加,加入0.1的蛋白质使蛋白质重新折叠。 M的NaCl。通过分别在385和280 nm处选择性激发血红素和色氨酸(Trp)来测量复合物的飞秒瞬态吸收光谱。尽管在385 nm激发下,血红素在脂质体的激发态弛豫动力学中未观察到显着变化,280 nm激发表现出系统的激发态弛豫动力学的系统性增加,从而导致Trp的寿命增加以及整体构象弛豫动力学随着脂质阴离子电荷的增加而增加。这揭示了由于脂质体使蛋白质的部分展开增加时,由于从Trp到血红素的荧光共振能量转移的效率降低,这是由于它们之间的距离增加。这样的观察显示出Trp作为标记氨基酸,以反映由于脂质体中血红素蛋白的三级结构的变化和轴向配体相互作用而引起的蛋白部分展开的动力学。在盐的存在下,复合物的弛豫动力学与单独的蛋白质相似,
更新日期:2020-03-27
中文翻译:

超快血红素松弛动力学探测脂质体诱导的细胞色素c的未折叠状态:磷脂电荷的影响
普遍存在的电子转移血红素蛋白,细胞色素c(Cyt c)在细胞凋亡的早期催化心磷脂(CL)的过氧化,其中Cyt c发生构象变化,导致该蛋白的部分展开。Cyt c与具有不同电荷的脂质体的相互作用动力学[CL,– 2; POPG(2-油酰基-1-棕榈酰基-sn-甘油-3-磷酸-rac-(1-甘油)钠盐),-1; 和POPC(2-油酰基-1-棕榈酰基-sn-glycero-3-phosphocholine),0]用稳态光谱和飞秒时间分辨泵浦-探针光谱研究了导致不同程度的部分展开的部分。在Cyt c-脂质体复合物的吸收,荧光和CD光谱中观察到了蛋白质部分解开的特征,随着脂质/蛋白质(L / P)比的增加,加入0.1的蛋白质使蛋白质重新折叠。 M的NaCl。通过分别在385和280 nm处选择性激发血红素和色氨酸(Trp)来测量复合物的飞秒瞬态吸收光谱。尽管在385 nm激发下,血红素在脂质体的激发态弛豫动力学中未观察到显着变化,280 nm激发表现出系统的激发态弛豫动力学的系统性增加,从而导致Trp的寿命增加以及整体构象弛豫动力学随着脂质阴离子电荷的增加而增加。这揭示了由于脂质体使蛋白质的部分展开增加时,由于从Trp到血红素的荧光共振能量转移的效率降低,这是由于它们之间的距离增加。这样的观察显示出Trp作为标记氨基酸,以反映由于脂质体中血红素蛋白的三级结构的变化和轴向配体相互作用而引起的蛋白部分展开的动力学。在盐的存在下,复合物的弛豫动力学与单独的蛋白质相似,











































 京公网安备 11010802027423号
京公网安备 11010802027423号