当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Folic Acid-Modified Nanoerythrocyte for Codelivery of Paclitaxel and Tariquidar to Overcome Breast Cancer Multidrug Resistance.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.molpharmaceut.9b01148 Ping Zhong 1 , Xuehong Chen 1 , Rishuo Guo 1 , Xiaomei Chen 2 , Zhihao Chen 1 , Cui Wei 1 , Yusheng Li 1 , Wanting Wang 1 , Yi Zhou 3 , Linghao Qin 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.molpharmaceut.9b01148 Ping Zhong 1 , Xuehong Chen 1 , Rishuo Guo 1 , Xiaomei Chen 2 , Zhihao Chen 1 , Cui Wei 1 , Yusheng Li 1 , Wanting Wang 1 , Yi Zhou 3 , Linghao Qin 1
Affiliation
|
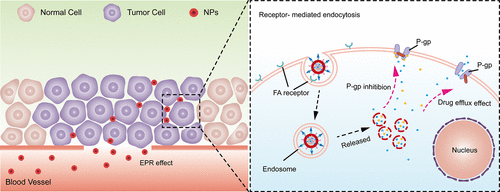
The efflux of anticancer agents mediated by P-glycoprotein (P-gp) is one of the main causes of multidrug resistance (MDR) and eventually leads to chemotherapy failure. To overcome this problem, the delivery of anticancer agents in combination with a P-gp inhibitor using nanocarrier systems is considered an effective strategy. On the basis of the physiological compatibility and excellent drug loading ability of erythrocytes, we hypothesized that nanoerythrocytes could be used for the codelivery of an anticancer agent and a P-gp inhibitor to overcome MDR in breast cancer. Herein, a folic acid-modified nanoerythrocyte system (PTX/TQR NPs@NanoRBC-PEG/FA) was prepared to simultaneously transport paclitaxel and tariquidar, and the in vitro and in vivo characteristics of this delivery system were evaluated through several experiments. The results indicated that the average diameter and surface potential of this nanocarrier system were 159.8 ± 1.4 nm and −10.98 mV, respectively. Within 120 h, sustained release of paclitaxel was observed in both pH 6.5 media and pH 7.4 media. Tariquidar release from this nanocarrier suppressed the P-gp function of MCF-7/Taxol cells and significantly increased the intracellular paclitaxel level (p < 0.01 versus the PTX group). The results of the MTT assay indicated that the simultaneous transportation of paclitaxel and tariquidar could significantly inhibit the growth of MCF-7 cells or MCF-7/Taxol cells. After 48 h of incubation with PTX/TQR NPs@NanoRBC-PEG/FA, the viability of MCF-7 cells and MCF-7/Taxol cells decreased to 7.37% and 30.2%, respectively, and the IC50 values were 2.49 μM and 6.30 μM. Pharmacokinetic results illustrated that, compared with free paclitaxel, all test paclitaxel nanoformulations prolonged the drug release time and showed similar plasma concentration–time profiles. The peak concentration (Cmax), area under the curve (AUC0–∞), and half-life (t1/2) of PTX/TQR NPs@NanoRBC-PEG/FA were 3.33 mg/L, 6.02 mg/L·h, and 5.84 h, respectively. Moreover, this active targeting nanocarrier dramatically increased the paclitaxel level in tumor tissues. Furthermore, compared with those of the other paclitaxel formulations, the cellular reactive oxygen species (ROS) and malondialdehyde (MDA) levels of the PTX/TQR NPs@NanoRBC-PEG/FA group increased by 1.38-fold (p < 0.01) and 1.36-fold (p < 0.01), respectively, and the activities of superoxide dismutase (SOD) and catalase (CAT) decreased to 67.8% (p < 0.01) and 65.4% (p < 0.001), respectively. More importantly, in vivo antitumor efficacy results proved that the PTX/TQR NPs@NanoRBC-PEG/FA group exerted an outstanding tumor inhibition effect with no marked body weight loss and fewer adverse effects. In conclusion, by utilizing the inherent and advantageous properties of erythrocytes and surface modification strategies, this biomimetic targeted drug delivery system provides a promising platform for the codelivery of an anticancer agent and a P-gp inhibitor to treat MDR in breast cancer.
中文翻译:

叶酸修饰的纳米红细胞用于紫杉醇和塔利奎达的代码传递,以克服乳腺癌的多药耐药性。
P糖蛋白(P-gp)介导的抗癌药外排是多药耐药性(MDR)的主要原因之一,并最终导致化疗失败。为了克服这个问题,使用纳米载体系统将抗癌剂与P-gp抑制剂组合使用被认为是一种有效的策略。基于红细胞的生理相容性和出色的载药能力,我们假设纳米红细胞可用于抗癌剂和P-gp抑制剂的代码传递,以克服乳腺癌中的MDR。本文中,制备了叶酸修饰的纳米红细胞系统(PTX / TQR NPs @ NanoRBC-PEG / FA)以同时转运紫杉醇和塔利奎达,并通过几次实验评价了该递送系统的体外和体内特性。结果表明,该纳米载体系统的平均直径和表面电势分别为159.8±1.4 nm和-10.98 mV。在120小时内,在pH 6.5介质和pH 7.4介质中均观察到紫杉醇的持续释放。从这种纳米载体释放的塔利奎达抑制了MCF-7 / Taxol细胞的P-gp功能,并显着提高了细胞内紫杉醇的水平(与PTX组相比,p <0.01)。MTT分析的结果表明,紫杉醇和塔利奎达的同时转运可以显着抑制MCF-7细胞或MCF-7 / Taxol细胞的生长。与PTX / TQR NPs @ NanoRBC-PEG / FA孵育48小时后,MCF-7细胞和MCF-7 / Taxol细胞的活力分别降低至7.37%和30.2%,IC 50值为2.49μM和6.30微米 药代动力学结果表明,与游离紫杉醇相比,所有测试紫杉醇纳米制剂均能延长药物释放时间,并显示出相似的血浆浓度-时间曲线。峰浓度(C max),曲线下面积(AUC 0–∞)和半衰期(t 1/2PTX / TQR NPs @ NanoRBC-PEG / FA)分别为3.33 mg / L,6.02 mg / L·h和5.84 h。而且,这种活性靶向纳米载体显着提高了肿瘤组织中紫杉醇的水平。此外,与其他紫杉醇制剂相比,PTX / TQR NPs @ NanoRBC-PEG / FA组的细胞活性氧(ROS)和丙二醛(MDA)水平增加了1.38倍(p <0.01)和1.36倍数(p <0.01)和超氧化物歧化酶(SOD)和过氧化氢酶(CAT)的活性分别降至67.8%(p <0.01)和65.4%(p<0.001)。更重要的是,体内抗肿瘤功效的结果证明了PTX / TQR NPs @ NanoRBC-PEG / FA组发挥了杰出的肿瘤抑制作用,没有明显的体重减轻,不良反应也更少。总之,通过利用红细胞的固有和有利特性以及表面修饰策略,这种仿生靶向药物递送系统为抗癌剂和P-gp抑制剂的代码递送提供了一个有前途的平台,以治疗乳腺癌的MDR。
更新日期:2020-03-16
中文翻译:

叶酸修饰的纳米红细胞用于紫杉醇和塔利奎达的代码传递,以克服乳腺癌的多药耐药性。
P糖蛋白(P-gp)介导的抗癌药外排是多药耐药性(MDR)的主要原因之一,并最终导致化疗失败。为了克服这个问题,使用纳米载体系统将抗癌剂与P-gp抑制剂组合使用被认为是一种有效的策略。基于红细胞的生理相容性和出色的载药能力,我们假设纳米红细胞可用于抗癌剂和P-gp抑制剂的代码传递,以克服乳腺癌中的MDR。本文中,制备了叶酸修饰的纳米红细胞系统(PTX / TQR NPs @ NanoRBC-PEG / FA)以同时转运紫杉醇和塔利奎达,并通过几次实验评价了该递送系统的体外和体内特性。结果表明,该纳米载体系统的平均直径和表面电势分别为159.8±1.4 nm和-10.98 mV。在120小时内,在pH 6.5介质和pH 7.4介质中均观察到紫杉醇的持续释放。从这种纳米载体释放的塔利奎达抑制了MCF-7 / Taxol细胞的P-gp功能,并显着提高了细胞内紫杉醇的水平(与PTX组相比,p <0.01)。MTT分析的结果表明,紫杉醇和塔利奎达的同时转运可以显着抑制MCF-7细胞或MCF-7 / Taxol细胞的生长。与PTX / TQR NPs @ NanoRBC-PEG / FA孵育48小时后,MCF-7细胞和MCF-7 / Taxol细胞的活力分别降低至7.37%和30.2%,IC 50值为2.49μM和6.30微米 药代动力学结果表明,与游离紫杉醇相比,所有测试紫杉醇纳米制剂均能延长药物释放时间,并显示出相似的血浆浓度-时间曲线。峰浓度(C max),曲线下面积(AUC 0–∞)和半衰期(t 1/2PTX / TQR NPs @ NanoRBC-PEG / FA)分别为3.33 mg / L,6.02 mg / L·h和5.84 h。而且,这种活性靶向纳米载体显着提高了肿瘤组织中紫杉醇的水平。此外,与其他紫杉醇制剂相比,PTX / TQR NPs @ NanoRBC-PEG / FA组的细胞活性氧(ROS)和丙二醛(MDA)水平增加了1.38倍(p <0.01)和1.36倍数(p <0.01)和超氧化物歧化酶(SOD)和过氧化氢酶(CAT)的活性分别降至67.8%(p <0.01)和65.4%(p<0.001)。更重要的是,体内抗肿瘤功效的结果证明了PTX / TQR NPs @ NanoRBC-PEG / FA组发挥了杰出的肿瘤抑制作用,没有明显的体重减轻,不良反应也更少。总之,通过利用红细胞的固有和有利特性以及表面修饰策略,这种仿生靶向药物递送系统为抗癌剂和P-gp抑制剂的代码递送提供了一个有前途的平台,以治疗乳腺癌的MDR。











































 京公网安备 11010802027423号
京公网安备 11010802027423号