当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroxylamines as Bifunctional Single-Nitrogen Sources for the Rapid Assembly of Diverse Tricyclic Indole Scaffolds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-17 , DOI: 10.1021/jacs.0c00403 Liangxin Fan 1 , Jiamao Hao 1 , Jingxun Yu 1 , Xiaojun Ma 1 , Jingjing Liu 1 , Xinjun Luan 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-17 , DOI: 10.1021/jacs.0c00403 Liangxin Fan 1 , Jiamao Hao 1 , Jingxun Yu 1 , Xiaojun Ma 1 , Jingjing Liu 1 , Xinjun Luan 1
Affiliation
|
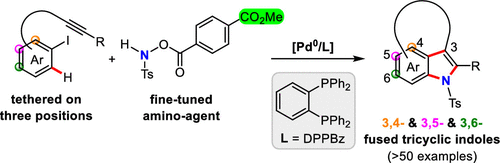
Conventional approaches on using hydroxylamine derivatives as single nitrogen sources, for the construction of n-membered (n>3) N-heterocycles, rely upon two chemical operations by involving sequential nucleophilic and electrophilic C-N bond formations. Here, we report a highly efficient cascade of alkyne insertion/C-H activation/amination for the rapid preparation of a myriad of tricyclic indoles, in a single-step transformation, by using bifunctional secondary hydroxylamines. Noteworthy, judicious selection of applicable amino agents, for enabling the prior oxidative addition of aryl iodide to initial Pd(0)-species and subsequent two C-N bonds formation, was the key to the success of this reaction. Control experiments indicated that five-membered palladacyclic intermediate played a crucial role in promoting the final aminative ring closure.
中文翻译:

羟胺作为双功能单氮源,用于多种三环吲哚支架的快速组装
使用羟胺衍生物作为单一氮源构建 n 元 (n>3) N-杂环的常规方法依赖于两个化学操作,涉及顺序亲核和亲电 CN 键的形成。在这里,我们报告了一种高效的炔插入/CH 活化/胺化级联,通过使用双功能仲羟胺,在一步转化中快速制备无数的三环吲哚。值得注意的是,明智地选择适用的氨基试剂,使芳基碘化物能够预先氧化加成到初始 Pd(0) 物种并随后形成两个 CN 键,是该反应成功的关键。对照实验表明,五元钯环中间体在促进最终胺化环闭合方面起着至关重要的作用。
更新日期:2020-03-17
中文翻译:

羟胺作为双功能单氮源,用于多种三环吲哚支架的快速组装
使用羟胺衍生物作为单一氮源构建 n 元 (n>3) N-杂环的常规方法依赖于两个化学操作,涉及顺序亲核和亲电 CN 键的形成。在这里,我们报告了一种高效的炔插入/CH 活化/胺化级联,通过使用双功能仲羟胺,在一步转化中快速制备无数的三环吲哚。值得注意的是,明智地选择适用的氨基试剂,使芳基碘化物能够预先氧化加成到初始 Pd(0) 物种并随后形成两个 CN 键,是该反应成功的关键。对照实验表明,五元钯环中间体在促进最终胺化环闭合方面起着至关重要的作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号