当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of β-lactams via diastereoselective, intramolecular Kinugasa reactions.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0ob00228c Oskar Popik 1 , Barbara Grzeszczyk 1 , Olga Staszewska-Krajewska 1 , Bartłomiej Furman 1 , Marek Chmielewski 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0ob00228c Oskar Popik 1 , Barbara Grzeszczyk 1 , Olga Staszewska-Krajewska 1 , Bartłomiej Furman 1 , Marek Chmielewski 1
Affiliation
|
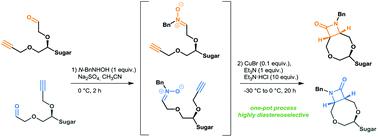
Intramolecular Kinugasa reactions on in situ generated carbohydrate-derived alkynylnitrones are described. The effects of the length of chains, their mutual configuration, influence of experimental conditions on product distribution and feasibility of the β-lactam ring construction were studied. Intramolecular reactions proceed with high stereoselectivity to provide in each case one product only. The cycloadducts from tartaric acid were converted into the corresponding non-racemic 4-acetoxy azetidinones in good yields.
中文翻译:

通过非对映选择性分子内Kinugasa反应合成β-内酰胺。
描述了在原位产生的碳水化合物衍生的炔基硝基酮上的分子内Kinugasa反应。研究了链长,互构,实验条件对产物分布的影响以及构建β-内酰胺环的可行性。分子内反应以高立体选择性进行,在每种情况下仅提供一种产物。来自酒石酸的环加合物以良好的产率转化为相应的非外消旋的4-乙酰氧基氮杂环丁烷酮。
更新日期:2020-04-24
中文翻译:

通过非对映选择性分子内Kinugasa反应合成β-内酰胺。
描述了在原位产生的碳水化合物衍生的炔基硝基酮上的分子内Kinugasa反应。研究了链长,互构,实验条件对产物分布的影响以及构建β-内酰胺环的可行性。分子内反应以高立体选择性进行,在每种情况下仅提供一种产物。来自酒石酸的环加合物以良好的产率转化为相应的非外消旋的4-乙酰氧基氮杂环丁烷酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号