Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-03-16 , DOI: 10.1038/s41419-020-2371-x Jie Ye 1, 2 , Anpei Huang 3 , Haitao Wang 1, 4 , Anni M Y Zhang 5 , Xiaojun Huang 1, 2 , Qingping Lan 1, 2 , Tomohiko Sato 6 , Susumu Goyama 6 , Mineo Kurokawa 6 , Chuxia Deng 1, 2 , Maike Sander 7 , David F Schaeffer 8 , Wen Li 3 , Janel L Kopp 5 , Ruiyu Xie 1, 2
|
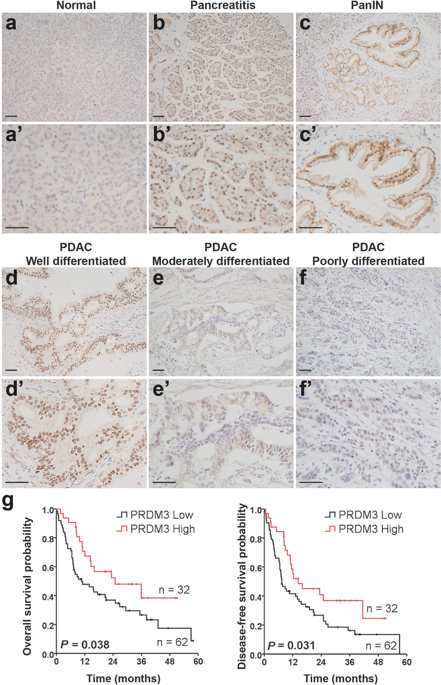
Pancreatic ductal adenocarcinoma (PDAC) is associated with metaplastic changes in the pancreas but the transcriptional program underlying these changes is incompletely understood. The zinc finger transcription factor, PRDM3, is lowly expressed in normal pancreatic acini and its expression increases during tumorigenesis. Although PRDM3 promotes proliferation and migration of PDAC cell lines, the role of PRDM3 during tumor initiation from pancreatic acinar cells in vivo is unclear. In this study, we showed that high levels of PRDM3 expression in human pancreas was associated with pancreatitis, and well-differentiated but not poorly differentiated carcinoma. We examined PRDM3 function in pancreatic acinar cells during tumor formation and pancreatitis by inactivating Prdm3 using a conditional allele (Ptf1aCreER;Prdm3flox/flox mice) in the context of oncogenic Kras expression and supraphysiological cerulein injections, respectively. In Prdm3-deficient mice, KrasG12D-driven preneoplastic lesions were more abundant and progressed to high-grade precancerous lesions more rapidly. This is consistent with our observations that low levels of PRDM3 in human PDAC was correlated significantly with poorer survival in patient. Moreover, loss of Prdm3 in acinar cells elevated exocrine injury, enhanced immune cell activation and infiltration, and greatly increased acinar-to-ductal cell reprogramming upon cerulein-induced pancreatitis. Whole transcriptome analyses of Prdm3 knockout acini revealed that pathways involved in inflammatory response and Hif-1 signaling were significantly upregulated in Prdm3-depleted acinar cells. Taken together, our results suggest that Prdm3 favors the maintenance of acinar cell homeostasis through modulation of their response to inflammation and oncogenic Kras activation, and thus plays a previously unexpected suppressive role during PDAC initiation.
中文翻译:

PRDM3 通过调节炎症反应减轻胰腺炎和胰腺肿瘤发生
胰腺导管腺癌(PDAC)与胰腺化生变化相关,但这些变化背后的转录程序尚不完全清楚。锌指转录因子 PRDM3 在正常胰腺腺泡中低表达,在肿瘤发生过程中表达增加。尽管PRDM3促进PDAC细胞系的增殖和迁移,但PRDM3在体内胰腺腺泡细胞肿瘤起始过程中的作用尚不清楚。在这项研究中,我们发现人胰腺中高水平的 PRDM3 表达与胰腺炎和高分化癌相关,但与低分化癌无关。我们分别在致癌Kras表达和超生理学雨蛙素注射的背景下,使用条件等位基因( Ptf1a CreER ;Prdm3 flox/flox小鼠)灭活Prdm3 ,检查了肿瘤形成和胰腺炎期间胰腺腺泡细胞中 PRDM3 的功能。在Prdm3缺陷小鼠中, Kras G12D驱动的癌前病变更加丰富,并且更快地进展为高级癌前病变。这与我们的观察结果一致,即人 PDAC 中 PRDM3 的低水平与患者较差的生存率显着相关。此外,腺泡细胞中Prdm3的缺失加剧了外分泌损伤,增强了免疫细胞的激活和浸润,并大大增加了雨蛙蛋白诱导的胰腺炎时腺泡到导管细胞的重编程。 Prdm3敲除腺泡的全转录组分析表明,在Prdm3缺失的腺泡细胞中,参与炎症反应和 Hif-1 信号传导的通路显着上调。综上所述,我们的结果表明, Prdm3通过调节腺泡细胞对炎症和致癌Kras激活的反应来维持腺泡细胞稳态,从而在 PDAC 启动过程中发挥先前意想不到的抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号