Nature Genetics ( IF 30.8 ) Pub Date : 2020-03-16 , DOI: 10.1038/s41588-020-0590-9 Kevin H Lin 1 , Justine C Rutter 1 , Abigail Xie 1 , Bryann Pardieu 2 , Emily T Winn 3 , Reinaldo Dal Bello 2, 4 , Antoine Forget 2 , Raphael Itzykson 2, 5 , Yeong-Ran Ahn 1 , Ziwei Dai 1 , Raiyan T Sobhan 1 , Gray R Anderson 1 , Katherine R Singleton 1 , Amy E Decker 1 , Peter S Winter 1 , Jason W Locasale 1 , Lorin Crawford 6 , Alexandre Puissant 2 , Kris C Wood 1
|
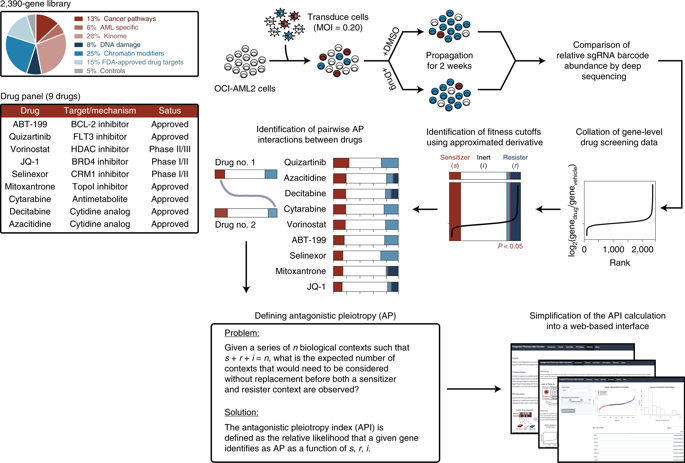
Local adaptation directs populations towards environment-specific fitness maxima through acquisition of positively selected traits. However, rapid environmental changes can identify hidden fitness trade-offs that turn adaptation into maladaptation, resulting in evolutionary traps. Cancer, a disease that is prone to drug resistance, is in principle susceptible to such traps. We therefore performed pooled CRISPR–Cas9 knockout screens in acute myeloid leukemia (AML) cells treated with various chemotherapies to map the drug-dependent genetic basis of fitness trade-offs, a concept known as antagonistic pleiotropy (AP). We identified a PRC2–NSD2/3-mediated MYC regulatory axis as a drug-induced AP pathway whose ability to confer resistance to bromodomain inhibition and sensitivity to BCL-2 inhibition templates an evolutionary trap. Across diverse AML cell-line and patient-derived xenograft models, we find that acquisition of resistance to bromodomain inhibition through this pathway exposes coincident hypersensitivity to BCL-2 inhibition. Thus, drug-induced AP can be leveraged to design evolutionary traps that selectively target drug resistance in cancer.
中文翻译:

使用拮抗多效性设计化疗诱导的进化陷阱以靶向癌症的耐药性。
局部适应通过获取积极选择的特征,将人群引向特定于环境的适应度最大值。然而,快速的环境变化可以识别隐藏的适应性权衡,将适应转变为适应不良,从而导致进化陷阱。癌症是一种容易产生耐药性的疾病,原则上容易受到这种陷阱的影响。因此,我们对接受各种化疗的急性髓性白血病 (AML) 细胞进行了合并的 CRISPR–Cas9 基因敲除筛选,以绘制适应性权衡的药物依赖性遗传基础,这一概念被称为拮抗多效性 (AP)。我们将 PRC2–NSD2/3 介导的 MYC 调节轴确定为药物诱导的 AP 通路,其赋予对溴结构域抑制的抗性和对 BCL-2 抑制的敏感性的能力模板进化陷阱。在不同的 AML 细胞系和患者来源的异种移植模型中,我们发现通过该途径获得对溴结构域抑制的抗性会同时暴露出对 BCL-2 抑制的超敏反应。因此,药物诱导的 AP 可用于设计选择性靶向癌症耐药性的进化陷阱。



























 京公网安备 11010802027423号
京公网安备 11010802027423号