Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-03-16 , DOI: 10.1038/s41589-020-0499-8 Forrest Z Bowling 1 , Christian M Salazar 2 , Justin A Bell 1 , Tahrima S Huq 1 , Michael A Frohman 2 , Michael V Airola 1
|
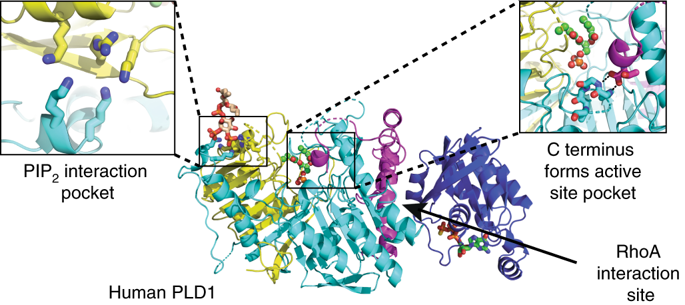
The signal transduction enzyme phospholipase D1 (PLD1) hydrolyzes phosphatidylcholine to generate the lipid second-messenger phosphatidic acid, which plays roles in disease processes such as thrombosis and cancer. PLD1 is directly and synergistically regulated by protein kinase C, Arf and Rho GTPases, and the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2). Here, we present a 1.8 Å-resolution crystal structure of the human PLD1 catalytic domain, which is characterized by a globular fold with a funnel-shaped hydrophobic cavity leading to the active site. Adjacent is a PIP2-binding polybasic pocket at the membrane interface that is essential for activity. The C terminus folds into and contributes part of the catalytic pocket, which harbors a phosphohistidine that mimics an intermediate stage of the catalytic cycle. Mapping of PLD1 mutations that disrupt RhoA activation identifies the RhoA-PLD1 binding interface. This structure sheds light on PLD1 regulation by lipid and protein effectors, enabling rationale inhibitor design for this well-studied therapeutic target.

中文翻译:

人类 PLD1 的晶体结构提供了对 PI(4,5)P2 和 RhoA 激活的洞察。
信号转导酶磷脂酶 D1 (PLD1) 水解磷脂酰胆碱生成脂质第二信使磷脂酸,在血栓形成和癌症等疾病过程中发挥作用。PLD1 受蛋白激酶 C、Arf 和 Rho GTP 酶以及膜脂 4,5-二磷酸磷脂酰肌醇 (PIP 2 ) 直接和协同调节。在这里,我们展示了人类 PLD1 催化结构域的 1.8 Å 分辨率晶体结构,其特征是具有通向活性位点的漏斗形疏水腔的球状折叠。相邻的是 PIP 2- 结合膜界面的多元口袋,对活性至关重要。C 末端折叠成催化袋并提供部分催化袋,该袋含有模拟催化循环中间阶段的磷酸组氨酸。破坏 RhoA 激活的 PLD1 突变的映射识别 RhoA-PLD1 结合界面。这种结构揭示了脂质和蛋白质效应器对 PLD1 的调节,从而为这个经过充分研究的治疗靶点设计合理的抑制剂。












































 京公网安备 11010802027423号
京公网安备 11010802027423号