当前位置:
X-MOL 学术
›
JAMA Pediatr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Association Between Proton Pump Inhibitor Use and Risk of Fracture in Children
JAMA Pediatrics ( IF 24.7 ) Pub Date : 2020-06-01 , DOI: 10.1001/jamapediatrics.2020.0007 Yun-Han Wang 1 , Viktor Wintzell 1 , Jonas F Ludvigsson 2, 3, 4, 5 , Henrik Svanström 1, 6 , Björn Pasternak 1, 6
JAMA Pediatrics ( IF 24.7 ) Pub Date : 2020-06-01 , DOI: 10.1001/jamapediatrics.2020.0007 Yun-Han Wang 1 , Viktor Wintzell 1 , Jonas F Ludvigsson 2, 3, 4, 5 , Henrik Svanström 1, 6 , Björn Pasternak 1, 6
Affiliation
|
Importance
Proton pump inhibitor (PPI) use has been linked to increased risk of fracture in adults. Despite a trend in prescription of PPIs in children, there is scarce evidence regarding this safety concern in pediatric patients. Objective
To evaluate the association between PPI use and risk of fracture in children. Design
This nationwide register-based cohort study included data from Sweden from July 2006 to December 2016. Children younger than 18 years who initiated PPI use were matched on propensity score and age with those who did not initiate PPI use. Exposure
Initiation of PPI use. Main Outcomes and Measures
Cox regression was adopted to estimate hazard ratios (HRs) for a first fracture of any type and 5 subtypes of fracture, with follow-up for up to 5 years. To address potential residual confounding, high-dimensional propensity score matching and a direct comparison with histamine-2 receptor antagonists were performed. Results
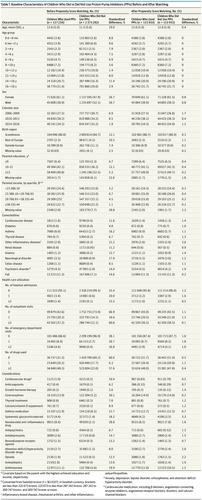
There were a total of 115 933 pairs of children included. During a mean (SD) of 2.2 (1.6) years of follow-up, 5354 and 4568 cases of any fracture occurred among those who initiated PPIs vs those who did not, respectively (20.2 vs 18.3 events per 1000 person-years; hazard ratio [HR], 1.11 [95% CI, 1.06-1.15]). Use of PPIs was associated with increased risk of upper-limb fracture (HR, 1.08 [95% CI, 1.03-1.13]), lower-limb fracture (HR, 1.19 [95% CI, 1.10-1.29]), and other fractures (HR, 1.51 [95% CI, 1.16-1.97]) but not head fracture (HR, 0.93 [95% CI, 0.76-1.13]) or spine fracture (HR, 1.31 [95% CI, 0.95-1.81]). The HRs for fracture according to cumulative duration of PPI use were 1.08 (95% CI, 1.03-1.13) for 30 days or less, 1.14 (95% CI, 1.09-1.20) for 31 to 364 days, and 1.34 (95% CI, 1.13-1.58) for 365 days or more. The association was consistent in most sensitivity analyses, including high-dimensional propensity score matching (HR, 1.10 [95% CI, 1.06-1.15]), although the analysis of PPI vs histamine-2 receptor antagonist did not reach statistical significance (HR, 1.06 [95% CI, 0.97-1.15]). Conclusions and Relevance
In this large pediatric cohort, PPI use was associated with a small but significant increased risk of any fracture. Risk of fracture should be taken into account when weighing the benefits and risks of PPI treatment in children.
中文翻译:

质子泵抑制剂使用与儿童骨折风险之间的关联
重要性 质子泵抑制剂 (PPI) 的使用与成人骨折风险增加有关。尽管在儿童中使用 PPI 有趋势,但很少有证据表明儿科患者存在这种安全问题。目的评价PPI使用与儿童骨折风险的关系。设计 这项基于登记的全国性队列研究包括 2006 年 7 月至 2016 年 12 月瑞典的数据。开始使用 PPI 的 18 岁以下儿童与未开始使用 PPI 的儿童在倾向评分和年龄方面进行匹配。暴露 PPI 使用的启动。主要结果和措施 采用 Cox 回归估计任何类型的首次骨折和 5 种骨折亚型的风险比 (HR),随访时间长达 5 年。为了解决潜在的残余混杂,进行了高维倾向评分匹配和与组胺-2受体拮抗剂的直接比较。结果共纳入115 933对儿童。在 2.2 (1.6) 年的平均 (SD) 随访期间,开始 PPI 的患者和未开始 PPI 的患者分别发生 5354 和 4568 例骨折(每 1000 人年 20.2 对 18.3 事件;风险比[HR],1.11 [95% CI,1.06-1.15])。使用 PPI 与上肢骨折(HR,1.08 [95% CI,1.03-1.13])、下肢骨折(HR,1.19 [95% CI,1.10-1.29])和其他骨折的风险增加相关(HR,1.51 [95% CI,1.16-1.97]),但不包括头部骨折(HR,0.93 [95% CI,0.76-1.13])或脊柱骨折(HR,1.31 [95% CI,0.95-1.81])。根据使用 PPI 的累积持续时间,骨折的 HR 为 1.08 (95% CI, 1.03-1. 13) 30 天或更短时间,1.14 (95% CI, 1.09-1.20) 31 至 364 天,1.34 (95% CI, 1.13-1.58) 365 天或更长时间。尽管 PPI 与组胺 2 受体拮抗剂的分析没有达到统计学意义(HR, 1.06 [95% CI,0.97-1.15])。结论和相关性 在这个大型儿科队列中,PPI 的使用与任何骨折风险的小幅但显着增加相关。在权衡儿童 PPI 治疗的益处和风险时,应考虑骨折风险。包括高维倾向评分匹配 (HR, 1.10 [95% CI, 1.06-1.15]),尽管 PPI 与组胺-2 受体拮抗剂的分析没有达到统计学意义 (HR, 1.06 [95% CI, 0.97-1.15] ])。结论和相关性 在这个大型儿科队列中,PPI 的使用与任何骨折风险的小幅但显着增加相关。在权衡儿童 PPI 治疗的益处和风险时,应考虑骨折风险。包括高维倾向评分匹配 (HR, 1.10 [95% CI, 1.06-1.15]),尽管 PPI 与组胺-2 受体拮抗剂的分析没有达到统计学意义 (HR, 1.06 [95% CI, 0.97-1.15] ])。结论和相关性 在这个大型儿科队列中,PPI 的使用与任何骨折风险的小幅但显着增加相关。在权衡儿童 PPI 治疗的益处和风险时,应考虑骨折风险。
更新日期:2020-06-01
中文翻译:

质子泵抑制剂使用与儿童骨折风险之间的关联
重要性 质子泵抑制剂 (PPI) 的使用与成人骨折风险增加有关。尽管在儿童中使用 PPI 有趋势,但很少有证据表明儿科患者存在这种安全问题。目的评价PPI使用与儿童骨折风险的关系。设计 这项基于登记的全国性队列研究包括 2006 年 7 月至 2016 年 12 月瑞典的数据。开始使用 PPI 的 18 岁以下儿童与未开始使用 PPI 的儿童在倾向评分和年龄方面进行匹配。暴露 PPI 使用的启动。主要结果和措施 采用 Cox 回归估计任何类型的首次骨折和 5 种骨折亚型的风险比 (HR),随访时间长达 5 年。为了解决潜在的残余混杂,进行了高维倾向评分匹配和与组胺-2受体拮抗剂的直接比较。结果共纳入115 933对儿童。在 2.2 (1.6) 年的平均 (SD) 随访期间,开始 PPI 的患者和未开始 PPI 的患者分别发生 5354 和 4568 例骨折(每 1000 人年 20.2 对 18.3 事件;风险比[HR],1.11 [95% CI,1.06-1.15])。使用 PPI 与上肢骨折(HR,1.08 [95% CI,1.03-1.13])、下肢骨折(HR,1.19 [95% CI,1.10-1.29])和其他骨折的风险增加相关(HR,1.51 [95% CI,1.16-1.97]),但不包括头部骨折(HR,0.93 [95% CI,0.76-1.13])或脊柱骨折(HR,1.31 [95% CI,0.95-1.81])。根据使用 PPI 的累积持续时间,骨折的 HR 为 1.08 (95% CI, 1.03-1. 13) 30 天或更短时间,1.14 (95% CI, 1.09-1.20) 31 至 364 天,1.34 (95% CI, 1.13-1.58) 365 天或更长时间。尽管 PPI 与组胺 2 受体拮抗剂的分析没有达到统计学意义(HR, 1.06 [95% CI,0.97-1.15])。结论和相关性 在这个大型儿科队列中,PPI 的使用与任何骨折风险的小幅但显着增加相关。在权衡儿童 PPI 治疗的益处和风险时,应考虑骨折风险。包括高维倾向评分匹配 (HR, 1.10 [95% CI, 1.06-1.15]),尽管 PPI 与组胺-2 受体拮抗剂的分析没有达到统计学意义 (HR, 1.06 [95% CI, 0.97-1.15] ])。结论和相关性 在这个大型儿科队列中,PPI 的使用与任何骨折风险的小幅但显着增加相关。在权衡儿童 PPI 治疗的益处和风险时,应考虑骨折风险。包括高维倾向评分匹配 (HR, 1.10 [95% CI, 1.06-1.15]),尽管 PPI 与组胺-2 受体拮抗剂的分析没有达到统计学意义 (HR, 1.06 [95% CI, 0.97-1.15] ])。结论和相关性 在这个大型儿科队列中,PPI 的使用与任何骨折风险的小幅但显着增加相关。在权衡儿童 PPI 治疗的益处和风险时,应考虑骨折风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号