当前位置:
X-MOL 学术
›
Arch. Biochem. Biophys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural analysis of a natural apolipoprotein A-I variant (L60R) associated with amyloidosis.
Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.abb.2020.108347 Gisela M Gaddi 1 , Romina A Gisonno 1 , Silvana A Rosú 1 , Lucrecia M Curto 2 , Eduardo D Prieto 3 , Guillermo R Schinella 4 , Gabriela S Finarelli 5 , M Fernanda Cortez 5 , Letizia Bauzá 5 , Esteban E Elías 5 , Nahuel A Ramella 1 , M Alejandra Tricerri 1
Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.abb.2020.108347 Gisela M Gaddi 1 , Romina A Gisonno 1 , Silvana A Rosú 1 , Lucrecia M Curto 2 , Eduardo D Prieto 3 , Guillermo R Schinella 4 , Gabriela S Finarelli 5 , M Fernanda Cortez 5 , Letizia Bauzá 5 , Esteban E Elías 5 , Nahuel A Ramella 1 , M Alejandra Tricerri 1
Affiliation
|
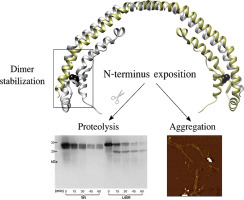
The reason that determines the pathological deposition of human apolipoprotein A-I variants inducing organ failure has been under research since the early description of natural mutations in patients. To shed light into the events associated with protein aggregation, we studied the structural perturbations that may occur in the natural variant that shows a substitution of a Leucine by an Arginine in position 60 (L60R). Circular dichroism, intrinsic fluorescence measurements, and proteolysis analysis indicated that L60R was more unstable, more sensitive to cleavage and the N-terminus was more disorganized than the protein with the native sequence (Wt). A higher tendency to aggregate was also detected when L60R was incubated at physiological pH. In addition, the small structural rearrangement observed for the freshly folded variant led to the release of tumor necrosis factor-α and interleukin-1β from a model of macrophages. However, the mutant preserved both its dimeric conformation and its lipid-binding capacity. Our results strongly suggest that the chronic disease may be a consequence of the native conformation loss which elicits the release of protein conformations that could be either cytotoxic or precursors of amyloid conformations.
中文翻译:

与淀粉样变性有关的天然载脂蛋白AI变体(L60R)的结构分析。
自从对患者自然突变的早期描述以来,就一直在研究确定导致人类器官衰竭的人类载脂蛋白AI变体的病理性沉积的原因。为了阐明与蛋白质聚集相关的事件,我们研究了自然变异中可能发生的结构扰动,该变异表明亮氨酸被位置60(L60R)的精氨酸取代。圆二色性,内在荧光测量和蛋白水解分析表明,与具有天然序列(Wt)的蛋白质相比,L60R更不稳定,对裂解更敏感,N末端更混乱。当L60R在生理pH下孵育时,也发现了更高的聚集趋势。此外,对新折叠的变体观察到的微小结构重排导致巨噬细胞模型释放肿瘤坏死因子-α和白介素-1β。但是,该突变体既保留了其二聚体构象,又保留了其脂质结合能力。我们的结果有力地表明,慢性疾病可能是天然构象丧失的结果,天然构象丧失引起蛋白质构象的释放,蛋白质构象可能是细胞毒性的,也可能是淀粉样蛋白构象的前体。
更新日期:2020-03-16
中文翻译:

与淀粉样变性有关的天然载脂蛋白AI变体(L60R)的结构分析。
自从对患者自然突变的早期描述以来,就一直在研究确定导致人类器官衰竭的人类载脂蛋白AI变体的病理性沉积的原因。为了阐明与蛋白质聚集相关的事件,我们研究了自然变异中可能发生的结构扰动,该变异表明亮氨酸被位置60(L60R)的精氨酸取代。圆二色性,内在荧光测量和蛋白水解分析表明,与具有天然序列(Wt)的蛋白质相比,L60R更不稳定,对裂解更敏感,N末端更混乱。当L60R在生理pH下孵育时,也发现了更高的聚集趋势。此外,对新折叠的变体观察到的微小结构重排导致巨噬细胞模型释放肿瘤坏死因子-α和白介素-1β。但是,该突变体既保留了其二聚体构象,又保留了其脂质结合能力。我们的结果有力地表明,慢性疾病可能是天然构象丧失的结果,天然构象丧失引起蛋白质构象的释放,蛋白质构象可能是细胞毒性的,也可能是淀粉样蛋白构象的前体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号