当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient synthesis of β-lactam antibiotics with in situ product removal by a newly isolated penicillin G acylase.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.bioorg.2020.103765 Xin Pan 1 , Anni Li 2 , Zhiyi Peng 3 , Xiaoqi Ji 3 , Jianlin Chu 2 , Bingfang He 2
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.bioorg.2020.103765 Xin Pan 1 , Anni Li 2 , Zhiyi Peng 3 , Xiaoqi Ji 3 , Jianlin Chu 2 , Bingfang He 2
Affiliation
|
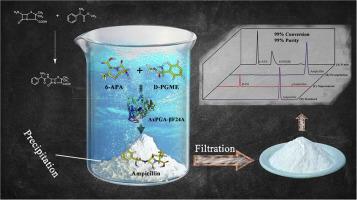
A penicillin G acylase (PGA) from Achromobacter xylosoxidans PX02 was newly isolated, and site-directed mutagenesis at three important positions αR141, αF142, βF24 was carried out for improving the enzymatic synthesis of β-lactam antibiotics. The efficient mutant βF24A was selected, and the (Ps/Ph)ini (ratio between the initial rate of synthesis and hydrolysis of the activated acyl donor) dramatically increased from 1.42-1.50 to 23.8-24.1 by means of the optimization of reaction conditions. Interestingly, the efficient enzymatic synthesis of ampicillin (99.1% conversion) and amoxicillin (98.7% conversion) from a high concentration (600 mM) of substrate 6-APA in the low acyl donor/nucleus ratio (1.1:1) resulted in a large amount of products precipitation from aqueous reaction solution. Meanwhile, the by-product D-phenylglycine was hardly precipitated, and 93.5% yield of precipitated ampicillin (561 mM) and 94.6% yield of precipitated amoxicillin (568 mM) were achieved with high purity (99%), which significantly simplified the downstream purification. This was the first study to achieve efficient β-lactam antibiotics synthesis process with in situ product removal, with barely any by-product formation. The effect enzymatic synthesis of antibiotics in aqueous reaction solution with in situ product removal provides a promising model for the industrial semi-synthesis of β-lactam antibiotics.
中文翻译:

通过新分离的青霉素G酰基转移酶原位去除产物,可高效合成β-内酰胺类抗生素。
从木氧化无色杆菌PX02中新分离出青霉素G酰基转移酶,并在三个重要位置αR141,αF142,βF24进行定点诱变,以改善β-内酰胺类抗生素的酶促合成。选择有效的突变体βF24A,并且通过优化反应条件,(Ps / Ph)ini(初始合成速率与活化的酰基供体的水解之间的比率)从1.42-1.50急剧增加至23.8-24.1。有趣的是,在低酰基供体/核比(1.1:1)下,由高浓度(600 mM)的底物6-APA有效地酶促合成氨苄青霉素(转化率为99.1%)和阿莫西林(转化率为98.7%)导致了一定数量的产物从反应水溶液中沉淀出来。与此同时,副产物D-苯基甘氨酸几乎不沉淀,高纯度(99%)的沉淀氨苄青霉素(561 mM)的产率为93.5%,阿莫西林沉淀的(568 mM)的产率为94.6%,显着简化了下游纯化。这是第一项通过原位去除产物而几乎不形成副产物的高效β-内酰胺类抗生素合成工艺的研究。反应水溶液中酶的酶促合成及原位产物去除效果为工业上半合成β-内酰胺类抗生素提供了一个有希望的模型。这是第一项通过原位去除产物而几乎不形成副产物的高效β-内酰胺类抗生素合成工艺的研究。反应水溶液中酶的酶促合成及原位产物去除效果为工业上半合成β-内酰胺类抗生素提供了一个有希望的模型。这是第一项通过原位去除产物而几乎不形成副产物的高效β-内酰胺类抗生素合成工艺的研究。反应水溶液中酶的酶促合成及原位产物去除效果为工业上半合成β-内酰胺类抗生素提供了有希望的模型。
更新日期:2020-04-20
中文翻译:

通过新分离的青霉素G酰基转移酶原位去除产物,可高效合成β-内酰胺类抗生素。
从木氧化无色杆菌PX02中新分离出青霉素G酰基转移酶,并在三个重要位置αR141,αF142,βF24进行定点诱变,以改善β-内酰胺类抗生素的酶促合成。选择有效的突变体βF24A,并且通过优化反应条件,(Ps / Ph)ini(初始合成速率与活化的酰基供体的水解之间的比率)从1.42-1.50急剧增加至23.8-24.1。有趣的是,在低酰基供体/核比(1.1:1)下,由高浓度(600 mM)的底物6-APA有效地酶促合成氨苄青霉素(转化率为99.1%)和阿莫西林(转化率为98.7%)导致了一定数量的产物从反应水溶液中沉淀出来。与此同时,副产物D-苯基甘氨酸几乎不沉淀,高纯度(99%)的沉淀氨苄青霉素(561 mM)的产率为93.5%,阿莫西林沉淀的(568 mM)的产率为94.6%,显着简化了下游纯化。这是第一项通过原位去除产物而几乎不形成副产物的高效β-内酰胺类抗生素合成工艺的研究。反应水溶液中酶的酶促合成及原位产物去除效果为工业上半合成β-内酰胺类抗生素提供了一个有希望的模型。这是第一项通过原位去除产物而几乎不形成副产物的高效β-内酰胺类抗生素合成工艺的研究。反应水溶液中酶的酶促合成及原位产物去除效果为工业上半合成β-内酰胺类抗生素提供了一个有希望的模型。这是第一项通过原位去除产物而几乎不形成副产物的高效β-内酰胺类抗生素合成工艺的研究。反应水溶液中酶的酶促合成及原位产物去除效果为工业上半合成β-内酰胺类抗生素提供了有希望的模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号