当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Local delivery of cardiac stem cells overexpressing HIF-1α promotes angiogenesis and muscular tissue repair in a hind limb ischemia model.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.jconrel.2020.03.017 Xing Pei 1 , Heejung Kim 2 , Minjoo Lee 2 , Nana Wang 1 , Jiyoung Shin 2 , Seungjin Lee 2 , Miyun Yoon 3 , Victor C Yang 4 , Huining He 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.jconrel.2020.03.017 Xing Pei 1 , Heejung Kim 2 , Minjoo Lee 2 , Nana Wang 1 , Jiyoung Shin 2 , Seungjin Lee 2 , Miyun Yoon 3 , Victor C Yang 4 , Huining He 1
Affiliation
|
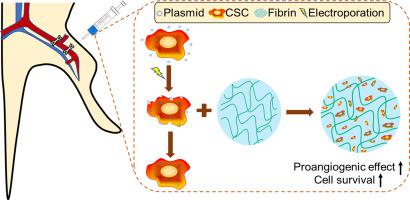
Critical limb ischemia (CLI) is the most advanced stage of peripheral artery disease, associated with significant risk of limb loss, morbidity and mortality; however, there remains unmet therapeutic needs for arterial revascularization and ischemic tissue repair. Stem cell therapies have emerged as compelling candidates due to beneficial proangiogenic and immunosuppressive function. Nevertheless, in vivo efficacy was insufficient in proliferation, differentiation and survival/engraftment rate. Cardiac stem cells (CSCs) was firstly attempted for CLI as a novel therapeutic modality to provide superior angiogenic potency to bone marrow-derived stem cells (BMSCs). It was noted that CSCs demonstrated 3.2-fold in HGF, 2.9-fold in VEGF and 8.7-fold in PDGF-B higher gene expressions compared to BMSCs. To enhance the hypoxia-induced proangiogenic effect, CSCs were transfected with hypoxia-inducible factor-1 alpha (HIF-1α) by using electroporation method, specifically optimized for CSCs yielding 45.77% of transfection efficiency and 89.75% of viability. HIF-1α overexpression significantly increased CSC survival in hypoxia, proangiogenic factors production and endothelial differentiation. In mouse hind limb ischemia model, local intramuscular delivery of CSC overexpressing HIF-1α (HIF-CSC) significantly improved the blood flow recovery. Histological analysis revealed that muscle degeneration and fibrosis in the ischemic limb were attenuated. Local delivery of HIF-CSC might be a promising option for ischemic tissue restoration.
中文翻译:

在后肢缺血模型中,过表达HIF-1α的心脏干细胞的局部递送促进血管生成和肌肉组织修复。
严重肢体缺血(CLI)是外周动脉疾病的最晚期,与肢体丢失,发病率和死亡率的重大风险相关;然而,动脉血运重建和缺血性组织修复的治疗需求仍未得到满足。由于有益的促血管生成和免疫抑制功能,干细胞疗法已成为令人信服的候选药物。然而,体内功效在增殖,分化和存活/植入率方面不足。首次尝试将心脏干细胞(CSCs)用于CLI,作为一种新颖的治疗方式,以提供优于骨髓衍生干细胞(BMSCs)的血管生成效能。值得注意的是,与BMSC相比,CSC在HGF中的基因表达高出3.2倍,在VEGF中是2.9倍,在PDGF-B中是8.7倍。为了增强缺氧诱导的血管生成作用,通过电穿孔方法,用缺氧诱导因子-1α(HIF-1α)转染CSC,该方法专门针对CSCs优化,可产生45.77%的转染效率和89.75%的活力。HIF-1α的过表达显着增加了缺氧,促血管生成因子的产生和内皮细胞分化过程中CSC的存活率。在小鼠后肢缺血模型中,局部肌内递送CSC过表达的HIF-1α(HIF-CSC)可显着改善血流恢复。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。转染效率为77%,生存力为89.75%。HIF-1α的过表达显着增加了缺氧,促血管生成因子产生和内皮分化过程中CSC的存活率。在小鼠后肢缺血模型中,局部肌内递送CSC过表达的HIF-1α(HIF-CSC)可以显着改善血流恢复。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。转染效率为77%,生存力为89.75%。HIF-1α的过表达显着增加了缺氧,促血管生成因子产生和内皮分化过程中CSC的存活率。在小鼠后肢缺血模型中,局部肌内递送CSC过表达的HIF-1α(HIF-CSC)可显着改善血流恢复。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。
更新日期:2020-04-21
中文翻译:

在后肢缺血模型中,过表达HIF-1α的心脏干细胞的局部递送促进血管生成和肌肉组织修复。
严重肢体缺血(CLI)是外周动脉疾病的最晚期,与肢体丢失,发病率和死亡率的重大风险相关;然而,动脉血运重建和缺血性组织修复的治疗需求仍未得到满足。由于有益的促血管生成和免疫抑制功能,干细胞疗法已成为令人信服的候选药物。然而,体内功效在增殖,分化和存活/植入率方面不足。首次尝试将心脏干细胞(CSCs)用于CLI,作为一种新颖的治疗方式,以提供优于骨髓衍生干细胞(BMSCs)的血管生成效能。值得注意的是,与BMSC相比,CSC在HGF中的基因表达高出3.2倍,在VEGF中是2.9倍,在PDGF-B中是8.7倍。为了增强缺氧诱导的血管生成作用,通过电穿孔方法,用缺氧诱导因子-1α(HIF-1α)转染CSC,该方法专门针对CSCs优化,可产生45.77%的转染效率和89.75%的活力。HIF-1α的过表达显着增加了缺氧,促血管生成因子的产生和内皮细胞分化过程中CSC的存活率。在小鼠后肢缺血模型中,局部肌内递送CSC过表达的HIF-1α(HIF-CSC)可显着改善血流恢复。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。转染效率为77%,生存力为89.75%。HIF-1α的过表达显着增加了缺氧,促血管生成因子产生和内皮分化过程中CSC的存活率。在小鼠后肢缺血模型中,局部肌内递送CSC过表达的HIF-1α(HIF-CSC)可以显着改善血流恢复。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。转染效率为77%,生存力为89.75%。HIF-1α的过表达显着增加了缺氧,促血管生成因子产生和内皮分化过程中CSC的存活率。在小鼠后肢缺血模型中,局部肌内递送CSC过表达的HIF-1α(HIF-CSC)可显着改善血流恢复。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。组织学分析显示,缺血肢体的肌肉变性和纤维化减弱。HIF-CSC的局部递送可能是缺血组织恢复的有前途的选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号