Dyes and Pigments ( IF 4.1 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.dyepig.2020.108370 Tomasz Koczorowski , Justyna Ber , Tomasz Sokolnicki , Anna Teubert , Wojciech Szczolko , Tomasz Goslinski
|
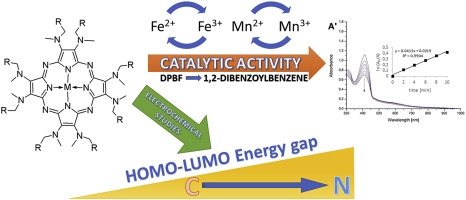
The cyclotetramerization reaction following the Linstead approach led to novel symmetrical magnesium(II) aminoporphyrazines with methyl(6-bromo-3-pyridylmethyl) and methyl(4-bromobenzyl) substituents. These products were applied in the demetallation reaction procedure in trifluoroacetic acid in the dark, which resulted in free-base porphyrazine derivatives. Then these macrocycles were remetallated with iron(II) bromide in DMF to give the desired iron(III) porphyrazine macrocycles. Simultaneously, the cyclotetramerization reaction of maleonitrile derivatives with manganese(II) chloride in n-pentanol and DBU led to symmetrical manganese(III) porphyrazines. New porphyrazine complexes with methyl(6-bromo-3-pyridylmethyl) and methyl(4-bromobenzyl) substituents were thoroughly characterized by the use of various analytical techniques, including electronic spectra, mass spectrometry, and FTIR. Macrocycles were subjected to electrochemical and spectroelectrochemical characterization, accompanied by preliminary catalytic study. The electrochemical properties of all obtained macrocycles were assessed with the use of cyclic and differential pulse voltammetry. The electrochemical activity of new macrocycles and their susceptibility to oxidation/reduction processes depended on the presence of nitrogen substituents in their macrocyclic periphery. The electrochemical measurements provided information on the positions of energy levels (HOMO-LUMO) of each porphyrazine, allowing estimation of the electrochemical energy gaps. The calculated electrochemical energy gaps were higher for porphyrazines with methyl(6-bromo-3-pyridylmethyl)amino peripheral substituents than methyl(4-bromobenzyl)amino ones due to the more electronegative nature of nitrogen amine atom. The calculated electrochemical HOMO-LUMO energy level gap values and the optical band gaps were found to agree within approx. 0.2 eV. Iron(III) and manganese(III) porphyrazines were assessed in terms of their catalytic properties. In these studies, DPBF was employed as a substrate of oxidation processes due to its easy UV–vis assessment. The results revealed the catalytic activity of porphyrazines with peripheral methyl(4-bromobenzyl)amino substituents following first-order kinetics.
中文翻译:

周围溴代芳基取代的锰和卟啉铁的电化学和催化评估
遵循Linstead方法的环四聚反应导致了具有甲基(6-溴-3-吡啶基甲基)和甲基(4-溴苄基)取代基的新型对称镁(II)氨基卟嗪。这些产物在黑暗中在三氟乙酸中用于脱金属反应过程中,产生了游离碱卟啉衍生物。然后将这些大环化合物在DMF中用溴化铁(II)重金属化,得到所需的铁(III)卟啉大环化合物。同时,马来腈衍生物与锰的cyclotetramerization反应(II)氯化物在Ñ-戊醇和DBU生成对称的锰(III)卟啉嗪。通过使用各种分析技术,包括电子光谱,质谱和FTIR,对具有甲基(6-溴-3-吡啶基甲基)和甲基(4-溴苄基)取代基的新型卟啉复合物进行了全面表征。对大环化合物进行了电化学和光谱电化学表征,并进行了初步的催化研究。使用循环和差分脉冲伏安法评估所有获得的大环化合物的电化学性质。新大环的电化学活性及其对氧化/还原过程的敏感性取决于其大环外围是否存在氮取代基。电化学测量可提供有关每种卟啉嗪能级(HOMO-LUMO)位置的信息,从而可以估算电化学能隙。由于氮胺原子具有更大的负电性,具有甲基(6-溴-3-吡啶基甲基)氨基外围取代基的卟啉比甲基(4-溴苄基)氨基具有更高的电化学能隙。发现计算出的电化学HOMO-LUMO能级能隙值和光学带隙在约10%内一致。0.2 eV。铁(III)和锰(III)卟啉嗪的催化性能得到了评估。在这些研究中,由于DPBF易于评估紫外线,因此被用作氧化过程的底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号