Energy Storage Materials ( IF 18.9 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.ensm.2020.03.016 Wei Zheng , Qiong Liu , Zhenyu Wang , Zhiliang Wu , Shuai Gu , Lujie Cao , Kaili Zhang , Jan Fransaer , Zhouguang Lu
|
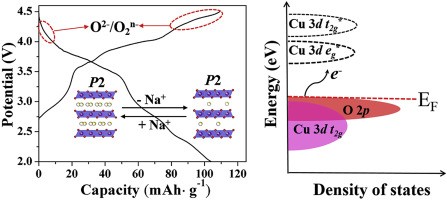
Although Li/Na layered oxides that display oxygen redox activity are promising cathodes with attractive energy density, they suffer from large voltage hysteresis and the evolution of O2. In this work, a layered Na0.67Cu0.28Mn0.72O2 cathode is reported that has reversible lattice oxygen redox activity with small voltage hysteresis. This compound provides a highly reversible capacity of 104 mAh g-1 with a smooth voltage profile originating from both cationic and anionic redox reactions. Density functional theory calculations show that the nonbonding O 2p states along the Cu–O bonds promote the oxygen redox activity. In-situ X-ray powder diffraction patterns and Raman spectra show that the small voltage hysteresis during electrochemical cycling is rooted in the absence of phase transitions and the stable oxygen stacking sequence. These findings may provide new insight into the anionic redox activity and offer a new strategy to design cathodes with high energy density and structural stability.
中文翻译:

Na 0.67 Cu 0.28 Mn 0.72 O 2中钠离子电池的低电压滞后氧还原活性
尽管显示出氧氧化还原活性的Li / Na层状氧化物有望成为具有诱人的能量密度的阴极,但它们具有较大的电压滞后和O 2的析出。在这项工作中,据报道层状Na 0.67 Cu 0.28 Mn 0.72 O 2阴极具有可逆的晶格氧氧化还原活性,且电压滞后小。该化合物可提供104 mAh g -1的高度可逆容量,并具有源自阳离子和阴离子氧化还原反应的平滑电压曲线。密度泛函理论计算表明,非键O 2 p沿Cu-O键的状态促进氧的氧化还原活性。原位X射线粉末衍射图谱和拉曼光谱表明,电化学循环过程中的小电压滞后现象是由于没有相变和稳定的氧堆积顺序而引起的。这些发现可能为阴离子氧化还原活性提供新的见解,并为设计具有高能量密度和结构稳定性的阴极提供了新的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号