当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crown ethers “clicked” on fibrous polyglycidyl methacrylate for selective Li+ retrieval from aqueous sources
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.colsurfa.2020.124709 Grace M. Nisola , Khino J. Parohinog , Rey Eliseo C. Torrejos , Sangho Koo , Seong-Poong Lee , Hern Kim , Wook-Jin Chung
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.colsurfa.2020.124709 Grace M. Nisola , Khino J. Parohinog , Rey Eliseo C. Torrejos , Sangho Koo , Seong-Poong Lee , Hern Kim , Wook-Jin Chung
|
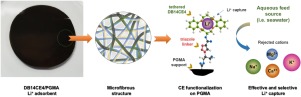
Abstract Increase in lithium (Li) demand has motivated the development of simple yet very effective materials for its recovery from aqueous resources. In this work, a microfibrous (MF) composite Li+ adsorbent tethered with dibenzo-14-crown-ether-4 (DB14CE4) as ligand was successfully fabricated. Polyglycidyl methacrylate (PGMA) was electrospun as MF support, rich with epoxy groups as reactive sites for DB14CE4 functionalization. PGMA MF was subsequently azidated (N3-PGMA) and covalently attached with alkyne terminated DB14CE4 (PPL-DB14CE4) via azide-alkyne cyclo-addition “click” chemistry. Characterization results confirm successful preparation of the final adsorbent DB14CE/PGMA MF. The Li+ adsorption mechanism is well described by Redlich-Peterson, Hill (nH ∼ 1) and Langmuir isotherm models with capacity qm ∼ 12.45 mg g−1. The Li+ uptake rate follows a pseudo-second order model. DB14CE4/PGMA MF preferentially captures Li+ in different aqueous sources (seawater, simulated rubber wastewater, simulated leachates from coal fly ash and lithium-ion battery waste). Li+ uptakes ranged qe ∼ 345–1428 μmol g−1 against competing ions (Na+, K+, Ba2+, Mg2+, Ca2+ Zn2+, Co2+, Cu2+, Al3+) having qe values
中文翻译:

冠醚“点击”纤维状聚甲基丙烯酸缩水甘油酯以从水源中选择性回收锂
摘要 锂 (Li) 需求的增加推动了开发简单但非常有效的材料以从水性资源中回收。在这项工作中,成功制造了一种以二苯并 14-冠醚-4 (DB14CE4) 作为配体的微纤维 (MF) 复合 Li+ 吸附剂。聚甲基丙烯酸缩水甘油酯 (PGMA) 作为 MF 载体进行电纺,富含环氧基团作为 DB14CE4 功能化的反应位点。PGMA MF 随后被叠氮化(N3-PGMA)并通过叠氮化物-炔烃环加成“点击”化学与炔烃封端的 DB14CE4(PPL-DB14CE4)共价连接。表征结果证实了最终吸附剂 DB14CE/PGMA MF 的成功制备。Redlich-Peterson、Hill (nH ∼ 1) 和容量 qm ∼ 12.45 mg g−1 的 Langmuir 等温线模型很好地描述了 Li+ 吸附机制。Li+ 吸收率遵循伪二阶模型。DB14CE4/PGMA MF 优先捕获不同水源(海水、模拟橡胶废水、粉煤灰和锂离子电池废物的模拟浸出液)中的 Li+。对于具有 qe 值的竞争离子(Na+、K+、Ba2+、Mg2+、Ca2+ Zn2+、Co2+、Cu2+、Al3+),Li+ 的吸收范围为 qe ∼ 345–1428 μmol g-1
更新日期:2020-07-01
中文翻译:

冠醚“点击”纤维状聚甲基丙烯酸缩水甘油酯以从水源中选择性回收锂
摘要 锂 (Li) 需求的增加推动了开发简单但非常有效的材料以从水性资源中回收。在这项工作中,成功制造了一种以二苯并 14-冠醚-4 (DB14CE4) 作为配体的微纤维 (MF) 复合 Li+ 吸附剂。聚甲基丙烯酸缩水甘油酯 (PGMA) 作为 MF 载体进行电纺,富含环氧基团作为 DB14CE4 功能化的反应位点。PGMA MF 随后被叠氮化(N3-PGMA)并通过叠氮化物-炔烃环加成“点击”化学与炔烃封端的 DB14CE4(PPL-DB14CE4)共价连接。表征结果证实了最终吸附剂 DB14CE/PGMA MF 的成功制备。Redlich-Peterson、Hill (nH ∼ 1) 和容量 qm ∼ 12.45 mg g−1 的 Langmuir 等温线模型很好地描述了 Li+ 吸附机制。Li+ 吸收率遵循伪二阶模型。DB14CE4/PGMA MF 优先捕获不同水源(海水、模拟橡胶废水、粉煤灰和锂离子电池废物的模拟浸出液)中的 Li+。对于具有 qe 值的竞争离子(Na+、K+、Ba2+、Mg2+、Ca2+ Zn2+、Co2+、Cu2+、Al3+),Li+ 的吸收范围为 qe ∼ 345–1428 μmol g-1



























 京公网安备 11010802027423号
京公网安备 11010802027423号