当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer.
Cancer Cell ( IF 48.8 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.ccell.2020.02.008 Samarth Hegde 1 , Varintra E Krisnawan 1 , Brett H Herzog 1 , Chong Zuo 1 , Marcus A Breden 1 , Brett L Knolhoff 1 , Graham D Hogg 1 , Jack P Tang 2 , John M Baer 1 , Cedric Mpoy 2 , Kyung Bae Lee 1 , Katherine A Alexander 1 , Buck E Rogers 3 , Kenneth M Murphy 4 , William G Hawkins 5 , Ryan C Fields 5 , Carl J DeSelm 3 , Julie K Schwarz 6 , David G DeNardo 7
Cancer Cell ( IF 48.8 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.ccell.2020.02.008 Samarth Hegde 1 , Varintra E Krisnawan 1 , Brett H Herzog 1 , Chong Zuo 1 , Marcus A Breden 1 , Brett L Knolhoff 1 , Graham D Hogg 1 , Jack P Tang 2 , John M Baer 1 , Cedric Mpoy 2 , Kyung Bae Lee 1 , Katherine A Alexander 1 , Buck E Rogers 3 , Kenneth M Murphy 4 , William G Hawkins 5 , Ryan C Fields 5 , Carl J DeSelm 3 , Julie K Schwarz 6 , David G DeNardo 7
Affiliation
|
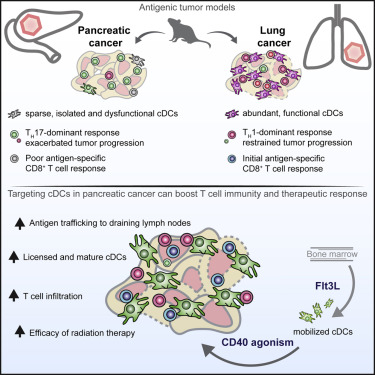
Here, we utilized spontaneous models of pancreatic and lung cancer to examine how neoantigenicity shapes tumor immunity and progression. As expected, neoantigen expression during lung adenocarcinoma development leads to T cell-mediated immunity and disease restraint. By contrast, neoantigen expression in pancreatic ductal adenocarcinoma (PDAC) results in exacerbation of a fibro-inflammatory microenvironment that drives disease progression and metastasis. Pathogenic TH17 responses are responsible for this neoantigen-induced tumor progression in PDAC. Underlying these divergent T cell responses in pancreas and lung cancer are differences in infiltrating conventional dendritic cells (cDCs). Overcoming cDC deficiency in early-stage PDAC leads to disease restraint, while restoration of cDC function in advanced PDAC restores tumor-restraining immunity and enhances responsiveness to radiation therapy.
中文翻译:

树突状细胞缺乏导致胰腺癌免疫功能异常。
在这里,我们利用胰腺和肺癌的自发模型来检查新抗原性如何塑造肿瘤的免疫力和进展。不出所料,在肺腺癌发生过程中新抗原的表达导致T细胞介导的免疫和疾病抑制。相比之下,胰腺导管腺癌(PDAC)中的新抗原表达导致驱动疾病进展和转移的纤维炎性微环境恶化。致病性TH17反应是PDAC中这种新抗原诱导的肿瘤进展的原因。这些在胰腺和肺癌中不同的T细胞反应的基础是浸润常规树突状细胞(cDC)的差异。克服早期PDAC中的cDC不足会导致疾病抑制,
更新日期:2020-03-16
中文翻译:

树突状细胞缺乏导致胰腺癌免疫功能异常。
在这里,我们利用胰腺和肺癌的自发模型来检查新抗原性如何塑造肿瘤的免疫力和进展。不出所料,在肺腺癌发生过程中新抗原的表达导致T细胞介导的免疫和疾病抑制。相比之下,胰腺导管腺癌(PDAC)中的新抗原表达导致驱动疾病进展和转移的纤维炎性微环境恶化。致病性TH17反应是PDAC中这种新抗原诱导的肿瘤进展的原因。这些在胰腺和肺癌中不同的T细胞反应的基础是浸润常规树突状细胞(cDC)的差异。克服早期PDAC中的cDC不足会导致疾病抑制,











































 京公网安备 11010802027423号
京公网安备 11010802027423号