Cancer Cell ( IF 48.8 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.ccell.2020.02.006 Hong Liu , Xinwei Kuang , Yongchang Zhang , Youqiong Ye , Jialu Li , Long Liang , Zuozhong Xie , Liang Weng , Jia Guo , Hui Li , Fangyu Ma , Xiaodan Chen , Shuang Zhao , Juan Su , Nong Yang , Fang Fang , Yang Xie , Juan Tao , Jianglin Zhang , Mingliang Chen , Cong Peng , Lunquan Sun , Xin Zhang , Jing Liu , Leng Han , Xiaowei Xu , Mien-Chie Hung , Xiang Chen
|
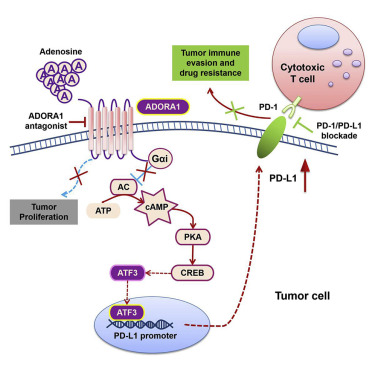
Here, we show that tumor ADORA1 deletion suppresses cell growth in human melanoma cell lines in vitro and tumor development in vivo in immune-deficient xenografts. However, this deletion induces the upregulation of PD-L1 levels, which inactivates cocultured T cells in vitro, compromises anti-tumor immunity in vivo, and reduces anti-tumor efficacy in an immune-competent mouse model. Functionally, PD-1 mAb treatment enhances the efficacy of ADORA1-deficient or ADORA1 antagonist-treated melanoma and NSCLC immune-competent mouse models. Mechanistically, we identify ATF3 as the factor transcriptionally upregulating PD-L1 expression. Tumor ATF3 deletion improves the effect of ADORA1 antagonist treatment of melanoma and NSCLC xenografts. We observe higher ADORA1, lower ATF3, and lower PD-L1 expression levels in tumor tissues from nonresponders among PD-1 mAb-treated NSCLC patients.
中文翻译:

ADORA1抑制通过调节ATF3-PD-L1轴促进肿瘤免疫逃逸。
这里,我们显示在人黑素瘤细胞系,肿瘤ADORA1删除禁止显示细胞生长的体外和肿瘤的发展在体内在免疫缺陷的异种移植物。但是,这种删除会诱导PD-L1水平的上调,从而在体外使共培养的T细胞失活,在体内损害抗肿瘤免疫力,并降低具有免疫功能的小鼠模型的抗肿瘤功效。从功能上讲,PD-1 mAb治疗可增强ADORA1的功效缺陷或ADORA1拮抗剂治疗的黑色素瘤和具有NSCLC免疫能力的小鼠模型。从机制上讲,我们将ATF3识别为转录上调PD-L1表达的因子。肿瘤ATF3缺失提高了ADORA1拮抗剂治疗黑素瘤和NSCLC异种移植物的效果。我们观察到PD-1 mAb治疗的NSCLC患者中无应答者的肿瘤组织中的ADORA1,更高的ATF3和更低的PD-L1表达水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号