European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.ejmech.2020.112235 Ying Wang , Xiaomei He , Chunshi Li , Yan Ma , Wenchi Xue , Baichun Hu , Jian Wang , Tingting Zhang , Fengjiao Zhang
|
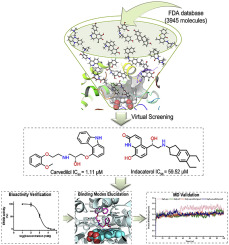
Cytochrome P450 1B1 (CYP1B1) is a promising target for prevention and therapy of cancer, particularly those with drug resistance, stimulating cancer cell survival, and promoting cancer resistance. In view of the extreme complexity and high risk in drug discovery and development, a drug repurposing strategy was applied in the present study to find potential CYP1B1 inhibitors through structure-based virtual screening in the FDA database. Intriguingly, after a thorough assessment of docking scores, binding affinities, as well as binding modes, six compounds were highlighted for further verification. In fact, both carvedilol and indacaterol showed inhibitory activity towards human CYP1B1 with the IC50 of 1.11 μM and 59.52 μM, respectively, according to EROD assay; however, neither docking score nor the detailed binding mode of carvedilol in the hit pose dictated to be a superior CYP1B1 inhibitor to indacaterol, which called for the necessity to re-access the binding mode of carvedilol. Thus, the top two representative docking poses of carvedilol were re-assessed. Indeed, compared to the one hit in the virtual screening (due to a false positive Glide gscore), the other docking pose exhibited ideal performance in both molecular dynamics (MD) simulation, binding free energy, and density functional theory (DFT) calculation evaluations. This identification of the exact binding pose of carvedilol is not only essential for a better understanding of the mechanism underlying its activity, but also contributes to uncovering the structure-activity relationship of CYP1B1 inhibitors. Of note, carvedilol exhibited direct cytotoxicity against both human lung adenocarcinoma epithelial cell line A459 and its Taxol-resistant subline (A549/Taxol). In particular, it showed superior toxicity towards A549/Taxol cells that overexpressed CYP1B1, which further supported its potential to be an effective CYP1B1 inhibitor.
中文翻译:

卡维地洛是一种新型的CYP1B1抑制剂,通过基于结构的虚拟筛选和实验验证实现了系统的药物重用
细胞色素P450 1B1(CYP1B1)是预防和治疗癌症的有希望的靶标,尤其是那些具有耐药性,刺激癌细胞存活并促进癌症抵抗力的癌症。鉴于药物发现和开发中的极端复杂性和高风险,本研究采用了药物重用策略,通过在FDA数据库中进行基于结构的虚拟筛选来发现潜在的CYP1B1抑制剂。有趣的是,在对对接分数,结合亲和力以及结合方式进行了全面评估之后,重点突出了六种化合物以进行进一步验证。实际上,卡维地洛和茚达特罗对IC 50均具有抑制人CYP1B1的活性。根据EROD分析分别为1.11μM和59.52μM; 然而,无论是对接得分还是卡维地洛在击中姿势中的详细结合方式均未表明其优于CYP1B1抑制剂茚达特罗,因此需要重新使用卡维地洛的结合方式。因此,重新评估了卡维地洛的前两个代表性对接姿势。实际上,与虚拟筛选中的一个命中结果(由于假阳性Glide gscore)相比,另一种对接姿势在分子动力学(MD)模拟,结合自由能和密度泛函理论(DFT)计算评估中均显示出理想的性能。 。对卡维地洛确切的结合姿势的识别不仅对于更好地了解其活性的基础机制至关重要,但同时也有助于揭示CYP1B1抑制剂的构效关系。值得注意的是,卡维地洛对人肺腺癌上皮细胞系A459及其抗紫杉醇的亚系(A549 / Taxol)均表现出直接的细胞毒性。特别是,它对过表达CYP1B1的A549 / Taxol细胞显示出优异的毒性,这进一步支持了其成为有效的CYP1B1抑制剂的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号