Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.bmcl.2020.127112 Rapolu Venkateshwarlu , Shambhu Nath Singh , Vidavalur Siddaiah , Hindupur Ramamohan , Rambabu Dandela , Kazi Amirul Hossain , P. Vijaya Babu , Manojit Pal
|
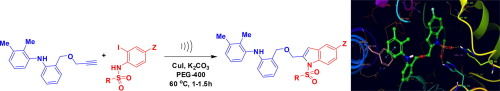
An improved and rapid synthesis of mefenamic acid based indole derivatives has been achieved via the ligand free Cu-catalyzed coupling-cyclization method under ultrasound irradiation. This simple, straightforward and inexpensive one-pot method involved the reaction of a terminal alkyne derived from mefenamic acid with 2-iodosulfanilides in the presence of CuI and K2CO3 in PEG-400. The reaction proceeded via an initial C-C bond formation (the coupling step) followed by C-N bond formation (the intramolecular cyclization) to afford the mefenamic acid based indole derivatives in good to acceptable yields. Several of these compounds showed inhibition of PDE4 in vitro and the SAR (Structure Activity Relationship) within the series is discussed. The compound 3d has been identified as a promising and selective inhibitor of PDE4B (IC50 = 1.34±0.46 µM) that showed TNF-α inhibition in vitro (IC50 = 5.81 ± 0.24 µM) and acceptable stability in the rat liver microsomes.
中文翻译:

超声辅助无配体铜催化下快速合成基于甲芬那酸的吲哚衍生物:药理评价
通过在超声辐射下无配体的铜催化的偶联-环化方法,已实现了基于甲芬那酸的吲哚衍生物的改进和快速合成。这种简单,直接和廉价的一锅法涉及在PEG-400中,在CuI和K 2 CO 3存在下,衍生自甲芬那酸的末端炔与2-碘磺酰苯胺的反应。该反应通过最初的CC键形成(偶联步骤)进行,然后通过CN键形成(分子内环化)进行,以良好的收率得到基于甲芬那酸的吲哚衍生物。这些化合物中的几种在体外显示出对PDE4的抑制作用并讨论了该系列中的SAR(结构活动关系)。化合物3d已被鉴定为PDE4B的有前途和选择性抑制剂(IC 50 = 1.34±0.46 µM),在体外显示出TNF-α抑制作用(IC 50 = 5.81±0.24 µM),并且在大鼠肝微粒体中具有可接受的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号