Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.tet.2020.131129 Shuntaro Sato , Yuka Taguchi , Shigefumi Kuwahara
|
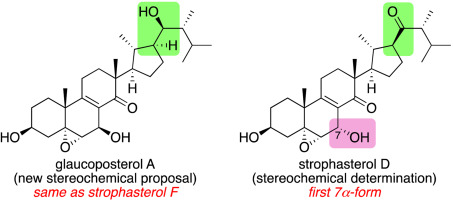
The proposed structure of glaucoposterol A, a strophastane-type steroid of mushroom origin, has been synthesized from an aldol obtained in our previous synthesis of strophasterol F, a diastereomer of the proposed structure of glaucoposterol A. Based on the fact that the NMR data of natural glaucoposterol A are distinctly different from those of the synthetic material, but matches those of strophasterol F, we put forward a proposal that glaucoposterol A and strophasterol F are actually the same compound. In addition, the hitherto unknown stereochemistry of strophasterol D has been elucidated through synthesis of its two diastereomeric structures.
中文翻译:

青铜固醇A和环丁固醇D的合成和立体化学
青霉素A的拟议结构是蘑菇来源的一种斯托伐他汀类固醇,是从我们以前合成的甾醇G的结构中的非对映异构体,苯酚甾醇F合成的醛醇中合成的。基于以下事实:天然青铜固醇A与合成材料明显不同,但与雌甾醇F相匹配,我们提出了一项建议,即古绿甾醇A和雌甾醇F实际上是同一化合物。另外,通过合成其两个非对映异构结构已经阐明了甾醇甾醇D的迄今未知的立体化学。










































 京公网安备 11010802027423号
京公网安备 11010802027423号