当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Determination, Model Correlation, and Solvent Effect Analysis of Nisoldipine in Different Solvent Systems at a Series of Temperature
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.jced.9b01036 Xia Chen 1 , Qianqian Xu 1 , Zhiying Liu 1 , Xianling Zhu 1 , Huilin Zheng 1 , Jia Zhao 2 , Rongrong Li 1 , Deman Han 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.jced.9b01036 Xia Chen 1 , Qianqian Xu 1 , Zhiying Liu 1 , Xianling Zhu 1 , Huilin Zheng 1 , Jia Zhao 2 , Rongrong Li 1 , Deman Han 1
Affiliation
|
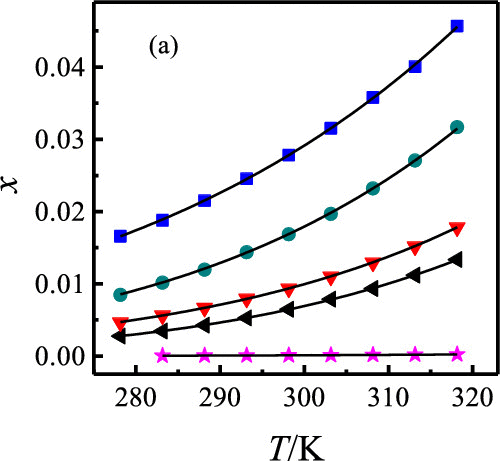
In this work, the solubility of nisoldipine in ethyl acetate, toluene, 1-butanol, 1-propanol, ethanol, acetonitrile, water, 2-propanol, cyclohexane, and two binary solvents mixtures (2-propanol/ethanol + water) at the temperature ranging from 278.15 to 318.15 K, solvent effect in a monosolvent system, and model correlation are studied. The solubility of nisoldipine is positively correlated with temperature. At a certain temperature, the subsequence of solubility of nisoldipine was of the order ethyl acetate (2.782 × 10–2, 298.15 K) > acetonitrile (1.687 × 10–2, 298.15 K) > 1-butanol (1.105 × 10–2, 298.15 K) > 2-propanol (9.350 × 10–3, 298.15 K) > 1-propanol (7.804 × 10–3, 298.15 K) > ethanol (6.483 × 10–3, 298.15 K) > toluene (3.092 × 10–3, 298.15 K) > cyclohexane (1.114 × 10–4, 298.15 K) > water (1.154 × 10–5, 298.15 K). To study the effect of solvation interaction on solubility, the solute–solvent and solvent–solvent interactions were studied. Hydrogen-bonding acceptor (HBA) interaction of the solvent with the solute and nonspecific dipolarity/polarizability interactions are in favor of the increase of solubility of nisoldipine. In addition, the solubility increases with an increasing composition of 2-propanol or ethanol and decreases with an increase of water. Four thermodynamic models (modified Apelblat model, λh model, Jouyban–Acree model, and Apelblat–Jouyban–Acree model) were used to correlate the relationships between the solubility of nisoldipine and temperature. The correlation results obtained from all systems show that the largest values of relative average deviation (RAD) and root-mean-square deviation (RMSD) are 1.35% and 1.13 × 10–4, respectively. Moreover, statistical analysis was used to evaluate the appropriateness of the models; in a word, these four models can describe the solubility behavior of nisoldipine very well.
中文翻译:

一系列温度下尼索地平在不同溶剂体系中的溶解度测定,模型相关性和溶剂效果分析
在这项工作中,尼索地平在乙酸乙酯,甲苯,1-丁醇,1-丙醇,乙醇,乙腈,水,2-丙醇,环己烷和两种二元溶剂混合物(2-丙醇/乙醇+水)中的溶解度研究了温度范围为278.15至318.15 K,单溶剂体系中的溶剂效应以及模型相关性。尼索地平的溶解度与温度呈正相关。在一定温度下,尼索地平的溶解性的子序列是顺序乙酸乙酯(2.782×10 -2,298.15 K)>乙腈(1.687×10 -2,298.15 K)> 1 -丁醇(1.105×10 -2, 298.15 K)> 2-丙醇(9.350×10 -3,298.15 K)> -1-丙醇(7.804×10 -3,298.15 K)>乙醇(6.483×10-3,298.15 K)>甲苯(3.092×10 -3,298.15 K)>环己烷(1.114×10 -4,298.15 K)>水(1.154×10 -5,298.15 K)。为了研究溶剂化相互作用对溶解度的影响,研究了溶质-溶剂和溶剂-溶剂的相互作用。溶剂与溶质的氢键受体(HBA)相互作用和非特异性偶极/极化性相互作用有利于尼索地平的溶解度增加。另外,溶解度随着2-丙醇或乙醇的组成的增加而增加,并且随着水的增加而减小。四个热力学模型(修改Apelblat模型,λ ^ h模型,Jouyban–Acree模型和Apelblat–Jouyban–Acree模型)用于关联尼索地平的溶解度与温度之间的关系。从所有系统获得的相关结果表明,相对平均偏差(RAD)和均方根偏差(RMSD)的最大值分别为1.35%和1.13×10 –4。此外,使用统计分析来评估模型的适用性。总之,这四个模型可以很好地描述尼索地平的溶解性行为。
更新日期:2020-04-24
中文翻译:

一系列温度下尼索地平在不同溶剂体系中的溶解度测定,模型相关性和溶剂效果分析
在这项工作中,尼索地平在乙酸乙酯,甲苯,1-丁醇,1-丙醇,乙醇,乙腈,水,2-丙醇,环己烷和两种二元溶剂混合物(2-丙醇/乙醇+水)中的溶解度研究了温度范围为278.15至318.15 K,单溶剂体系中的溶剂效应以及模型相关性。尼索地平的溶解度与温度呈正相关。在一定温度下,尼索地平的溶解性的子序列是顺序乙酸乙酯(2.782×10 -2,298.15 K)>乙腈(1.687×10 -2,298.15 K)> 1 -丁醇(1.105×10 -2, 298.15 K)> 2-丙醇(9.350×10 -3,298.15 K)> -1-丙醇(7.804×10 -3,298.15 K)>乙醇(6.483×10-3,298.15 K)>甲苯(3.092×10 -3,298.15 K)>环己烷(1.114×10 -4,298.15 K)>水(1.154×10 -5,298.15 K)。为了研究溶剂化相互作用对溶解度的影响,研究了溶质-溶剂和溶剂-溶剂的相互作用。溶剂与溶质的氢键受体(HBA)相互作用和非特异性偶极/极化性相互作用有利于尼索地平的溶解度增加。另外,溶解度随着2-丙醇或乙醇的组成的增加而增加,并且随着水的增加而减小。四个热力学模型(修改Apelblat模型,λ ^ h模型,Jouyban–Acree模型和Apelblat–Jouyban–Acree模型)用于关联尼索地平的溶解度与温度之间的关系。从所有系统获得的相关结果表明,相对平均偏差(RAD)和均方根偏差(RMSD)的最大值分别为1.35%和1.13×10 –4。此外,使用统计分析来评估模型的适用性。总之,这四个模型可以很好地描述尼索地平的溶解性行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号