当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfonimide and Amide Derivatives as Novel PPARα Antagonists: Synthesis, Antiproliferative Activity, and Docking Studies.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-03 , DOI: 10.1021/acsmedchemlett.9b00666 Alessandra Ammazzalorso 1 , Isabella Bruno 1 , Rosalba Florio 1 , Laura De Lellis 1 , Antonio Laghezza 2 , Carmen Cerchia 3 , Barbara De Filippis 1 , Marialuigia Fantacuzzi 1 , Letizia Giampietro 1 , Cristina Maccallini 1 , Paolo Tortorella 2 , Serena Veschi 1 , Fulvio Loiodice 2 , Antonio Lavecchia 3 , Alessandro Cama 1, 4 , Rosa Amoroso 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-03 , DOI: 10.1021/acsmedchemlett.9b00666 Alessandra Ammazzalorso 1 , Isabella Bruno 1 , Rosalba Florio 1 , Laura De Lellis 1 , Antonio Laghezza 2 , Carmen Cerchia 3 , Barbara De Filippis 1 , Marialuigia Fantacuzzi 1 , Letizia Giampietro 1 , Cristina Maccallini 1 , Paolo Tortorella 2 , Serena Veschi 1 , Fulvio Loiodice 2 , Antonio Lavecchia 3 , Alessandro Cama 1, 4 , Rosa Amoroso 1
Affiliation
|
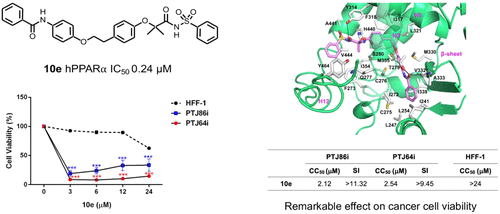
An agonist-antagonist switching strategy was performed to discover novel PPARα antagonists. Phenyldiazenyl derivatives of fibrates were developed, bearing sulfonimide or amide functional groups. A second series of compounds was synthesized, replacing the phenyldiazenyl moiety with amide or urea portions. Final compounds were screened by transactivation assay, showing good PPARα antagonism and selectivity at submicromolar concentrations. When tested in cancer cell models expressing PPARα, selected derivatives induced marked effects on cell viability. Notably, 3c, 3d, and 10e displayed remarkable antiproliferative effects in two paraganglioma cell lines, with CC50 lower than commercial PPARα antagonist GW6471 and a negligible toxicity on normal fibroblast cells. Docking studies were also performed to elucidate the binding mode of these compounds and to help interpretation of SAR data.
中文翻译:

磺酰亚胺和酰胺衍生物作为新型PPARα拮抗剂:合成,抗增殖活性和对接研究。
进行了激动剂-拮抗剂转换策略以发现新型的PPARα拮抗剂。已开发出带有磺酰亚胺或酰胺官能团的贝特类的苯二氮杂烯基衍生物。合成了第二系列的化合物,用酰胺或脲部分取代了苯基二氮烯基部分。通过反式激活试验筛选最终化合物,在亚微摩尔浓度下显示出良好的PPARα拮抗作用和选择性。在表达PPARα的癌细胞模型中进行测试时,选定的衍生物会诱导对细胞活力的显着影响。值得注意的是,3c,3d和10e在两种副神经节瘤细胞系中均表现出显着的抗增殖作用,其CC50低于商业PPARα拮抗剂GW6471,并且对正常成纤维细胞的毒性可忽略不计。
更新日期:2020-03-03
中文翻译:

磺酰亚胺和酰胺衍生物作为新型PPARα拮抗剂:合成,抗增殖活性和对接研究。
进行了激动剂-拮抗剂转换策略以发现新型的PPARα拮抗剂。已开发出带有磺酰亚胺或酰胺官能团的贝特类的苯二氮杂烯基衍生物。合成了第二系列的化合物,用酰胺或脲部分取代了苯基二氮烯基部分。通过反式激活试验筛选最终化合物,在亚微摩尔浓度下显示出良好的PPARα拮抗作用和选择性。在表达PPARα的癌细胞模型中进行测试时,选定的衍生物会诱导对细胞活力的显着影响。值得注意的是,3c,3d和10e在两种副神经节瘤细胞系中均表现出显着的抗增殖作用,其CC50低于商业PPARα拮抗剂GW6471,并且对正常成纤维细胞的毒性可忽略不计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号