当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthetic Incorporation of Site-Specific Isotopes in β-Lactam Antibiotics Enables Biophysical Studies.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-20 , DOI: 10.1021/acschembio.9b01054 Jacek Kozuch 1 , Samuel H Schneider 1 , Steven G Boxer 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-20 , DOI: 10.1021/acschembio.9b01054 Jacek Kozuch 1 , Samuel H Schneider 1 , Steven G Boxer 1
Affiliation
|
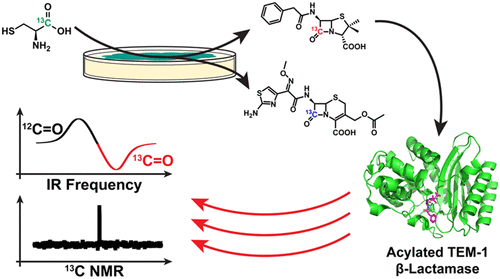
A biophysical understanding of the mechanistic, chemical, and physical origins underlying antibiotic action and resistance is vital to the discovery of novel therapeutics and the development of strategies to combat the growing emergence of antibiotic resistance. The site-specific introduction of stable-isotope labels into chemically complex natural products is particularly important for techniques such as NMR, IR, mass spectrometry, imaging, and kinetic isotope effects. Toward this goal, we developed a biosynthetic strategy for the site-specific incorporation of 13C labels into the canonical β-lactam carbonyl of penicillin G and cefotaxime, the latter via cephalosporin C. This was achieved through sulfur-replacement with 1-13C-l-cysteine, resulting in high isotope incorporations and milligram-scale yields. Using 13C NMR and isotope-edited IR difference spectroscopy, we illustrate how these molecules can be used to interrogate interactions with their protein targets, e.g., TEM-1 β-lactamase. This method provides a feasible route to isotopically labeled penicillin and cephalosporin precursors for future biophysical studies.
中文翻译:

特定位点同位素在β-内酰胺抗生素中的生物合成结合可进行生物物理研究。
对潜在的抗生素作用和耐药机制,化学和物理起源的生物物理理解对于发现新的疗法和对抗日益增长的耐药性的策略的发展至关重要。在化学复杂的天然产物中将位点特异性引入稳定同位素标记对于NMR,IR,质谱,成像和动力学同位素效应等技术尤为重要。为了实现这一目标,我们开发了一种生物合成策略,可将13C标记位点特异性结合到青霉素G和头孢噻肟的经典β-内酰胺羰基中,后者通过头孢菌素C来实现。这是通过用1-13C-1取代硫来实现的-半胱氨酸,导致高同位素掺入和毫克级收率。使用13 C NMR和同位素编辑的IR差异光谱,我们说明了如何使用这些分子来询问与其蛋白质靶标(例如TEM-1β-内酰胺酶)的相互作用。该方法为同位素标记的青霉素和头孢菌素前体提供了可行的途径,以用于未来的生物物理研究。
更新日期:2020-03-16
中文翻译:

特定位点同位素在β-内酰胺抗生素中的生物合成结合可进行生物物理研究。
对潜在的抗生素作用和耐药机制,化学和物理起源的生物物理理解对于发现新的疗法和对抗日益增长的耐药性的策略的发展至关重要。在化学复杂的天然产物中将位点特异性引入稳定同位素标记对于NMR,IR,质谱,成像和动力学同位素效应等技术尤为重要。为了实现这一目标,我们开发了一种生物合成策略,可将13C标记位点特异性结合到青霉素G和头孢噻肟的经典β-内酰胺羰基中,后者通过头孢菌素C来实现。这是通过用1-13C-1取代硫来实现的-半胱氨酸,导致高同位素掺入和毫克级收率。使用13 C NMR和同位素编辑的IR差异光谱,我们说明了如何使用这些分子来询问与其蛋白质靶标(例如TEM-1β-内酰胺酶)的相互作用。该方法为同位素标记的青霉素和头孢菌素前体提供了可行的途径,以用于未来的生物物理研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号