当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature-Dependent Kinetic Study of the Reaction of Hydroxyl Radicals with Hydroxyacetone
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jpca.0c00429 Yuri Bedjanian 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.jpca.0c00429 Yuri Bedjanian 1
Affiliation
|
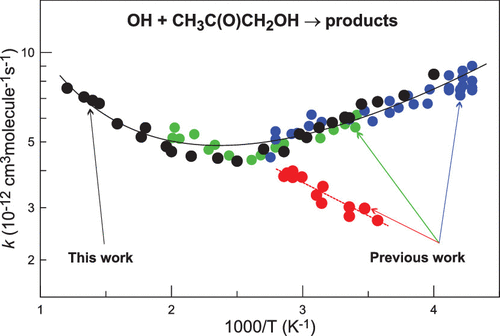
The kinetics of the reaction of OH radicals with hydroxyacetone has been investigated as a function of temperature at a total pressure of helium of 2.0–2.1 Torr and over an extended temperature range of T = 250–830 K and as a function of pressure at T = 301 K in the pressure range 1.0–10.4 Torr. The rate constant of the reaction OH + CH3C(O)CH2OH → products (1) was measured using both absolute (from the kinetics of OH consumption in excess of hydroxyacetone) and relative rate methods (k1 = 4.7 × 10–22 × T3.25 exp (1410/T) cm3 molecule–1 s–1 at T = 250–830 K). The present data combined with selected previous temperature-dependent studies of reaction (1) yield k1 = 4.4 × 10–20 × T2.63 exp (1110/T) cm3 molecule–1 s–1, which is recommended from the present work at T = 230–830 K (with conservative uncertainty of 20% at all temperatures). k1 was found to be independent of the pressure in the range from 1.0 to 10.4 Torr of He at T = 301 K. The present results are compared with previous experimental and theoretical data.
中文翻译:

羟基自由基与羟丙酮反应的温度依赖性动力学研究
OH自由基与羟基丙酮的反应动力学已被研究作为温度的函数的在2.0-2.1乇的氦的总压力和在扩展的温度范围内的Ť = 250-830 K和作为压力的函数Ť = 301 K,压力范围为1.0-10.4 Torr。OH + CH 3 C(O)CH 2 OH→产物(1)的反应速率常数使用绝对方法(根据过量羟基丙酮消耗OH的动力学)和相对速率方法(k 1 = 4.7×10)进行测量–22 × T 3.25 exp(1410 / T)cm 3分子–1 s –1在T = 250–830 K时)。本数据与先前选择的与温度有关的反应研究(1)结合,得出k 1 = 4.4×10 –20 × T 2.63 exp(1110 / T)cm 3分子–1 s –1,这是本工作推荐的在T = 230–830 K时(所有温度下的保守不确定度为20%)。发现在T = 301 K时,k 1与He的1.0至10.4 Torr范围内的压力无关。将当前结果与先前的实验和理论数据进行比较。
更新日期:2020-03-27
中文翻译:

羟基自由基与羟丙酮反应的温度依赖性动力学研究
OH自由基与羟基丙酮的反应动力学已被研究作为温度的函数的在2.0-2.1乇的氦的总压力和在扩展的温度范围内的Ť = 250-830 K和作为压力的函数Ť = 301 K,压力范围为1.0-10.4 Torr。OH + CH 3 C(O)CH 2 OH→产物(1)的反应速率常数使用绝对方法(根据过量羟基丙酮消耗OH的动力学)和相对速率方法(k 1 = 4.7×10)进行测量–22 × T 3.25 exp(1410 / T)cm 3分子–1 s –1在T = 250–830 K时)。本数据与先前选择的与温度有关的反应研究(1)结合,得出k 1 = 4.4×10 –20 × T 2.63 exp(1110 / T)cm 3分子–1 s –1,这是本工作推荐的在T = 230–830 K时(所有温度下的保守不确定度为20%)。发现在T = 301 K时,k 1与He的1.0至10.4 Torr范围内的压力无关。将当前结果与先前的实验和理论数据进行比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号