当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic Approaches to Phosphasugars (2-oxo-1,2-oxaphosphacyclanes) Using the Anomeric Alkoxyl Radical β-Fragmentation Reaction as the Key Step
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.joc.0c00059 Daniel Hernández-Guerra 1 , Alan R. Kennedy 2 , Elisa I. León 1 , Ángeles Martín 1 , Inés Pérez-Martín 1 , María S. Rodríguez 1 , Ernesto Suárez 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.joc.0c00059 Daniel Hernández-Guerra 1 , Alan R. Kennedy 2 , Elisa I. León 1 , Ángeles Martín 1 , Inés Pérez-Martín 1 , María S. Rodríguez 1 , Ernesto Suárez 1
Affiliation
|
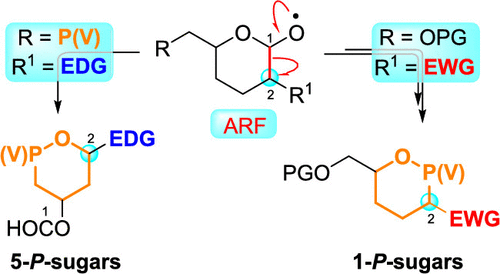
The anomeric alkoxyl radical β-fragmentation (ARF) of carbohydrates possessing an electron-withdrawing group (EWG) at C2, promoted by PhI(OAc)2/I2, gives rise to an acyclic iodide through which a pentavalent atom of phosphorus can be introduced via the Arbuzov reaction. After selective hydrolysis and subsequent cyclization, the phosphonate or phosphinate intermediates can be converted into 2-deoxy-1-phosphahexopyranose and 2-deoxy-1-phosphapentopyranose sugars. The ARF of carbohydrates with an electron-donor group (EDG) at C2 proceeds by a radical-polar crossover mechanism, and the cyclization occurs by nucleophilic attack of a conveniently positioned phosphonate or phosphinate group to the transient oxocarbenium ion. This alternative methodology leads to 5-phosphasugars with a 4-deoxy-5-phosphapentopyranose framework. The structure and conformation of the 2-oxo-1,2-oxaphosphinane and 2-oxo-1,2-oxaphospholane ring systems in different carbohydrate models have been studied by NMR and X-ray crystallography.
中文翻译:

端基烷氧基自由基β-片段化反应为关键步骤合成磷酸糖(2-oxo-1,2-oxaphosphacyclanes)的方法
PhI(OAc)2 / I 2促进的在C2具有吸电子基团(EWG)的碳水化合物的异头烷氧基自由基β片段化(ARF)产生无环碘化物,可通过Arbuzov反应将磷的五价原子引入其中。在选择性水解和随后的环化之后,可将膦酸酯或次膦酸酯中间体转化为2-脱氧-1-磷酸杂戊基吡喃糖和2-脱氧-1-磷酸戊烯基吡喃糖。在C2处具有电子给体基团(EDG)的碳水化合物的ARF通过自由基-极性交叉机制进行,并且环化反应是通过方便地定位的膦酸酯或次膦酸酯基团对瞬态氧碳鎓离子的亲核攻击而发生的。这种替代方法导致具有4-脱氧-5-磷酸戊烯基吡喃糖构架的5-磷酸戊糖。2-oxo-1,2-oxaphosphinane和2-oxo-1的结构和构象
更新日期:2020-03-26
中文翻译:

端基烷氧基自由基β-片段化反应为关键步骤合成磷酸糖(2-oxo-1,2-oxaphosphacyclanes)的方法
PhI(OAc)2 / I 2促进的在C2具有吸电子基团(EWG)的碳水化合物的异头烷氧基自由基β片段化(ARF)产生无环碘化物,可通过Arbuzov反应将磷的五价原子引入其中。在选择性水解和随后的环化之后,可将膦酸酯或次膦酸酯中间体转化为2-脱氧-1-磷酸杂戊基吡喃糖和2-脱氧-1-磷酸戊烯基吡喃糖。在C2处具有电子给体基团(EDG)的碳水化合物的ARF通过自由基-极性交叉机制进行,并且环化反应是通过方便地定位的膦酸酯或次膦酸酯基团对瞬态氧碳鎓离子的亲核攻击而发生的。这种替代方法导致具有4-脱氧-5-磷酸戊烯基吡喃糖构架的5-磷酸戊糖。2-oxo-1,2-oxaphosphinane和2-oxo-1的结构和构象









































 京公网安备 11010802027423号
京公网安备 11010802027423号