当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diels–Alder/Ene Reactivities of 2-(1′-Cycloalkenyl)thiophenes and 2-(1′-Cycloalkenyl)benzo[b]thiophenes with N-Phenylmaleimides: Role of Cycloalkene Ring Size on Benzothiophene and Dibenzothiophene Product Distributions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.joc.9b03363 Wayland E. Noland 1 , Honnaiah Vijay Kumar 1 , Yernaidu Reddi 1 , Christopher J. Cramer 1 , Alexei V. Novikov 1 , Hyejin Kim 1 , Yumeng Zhu 1 , Yoke Ching Chin 1 , Yuqi Zhou 1 , Predrag Radakovic 1 , Anjola Uprety 1 , Jun Xie 1 , Grant C. Flick 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.joc.9b03363 Wayland E. Noland 1 , Honnaiah Vijay Kumar 1 , Yernaidu Reddi 1 , Christopher J. Cramer 1 , Alexei V. Novikov 1 , Hyejin Kim 1 , Yumeng Zhu 1 , Yoke Ching Chin 1 , Yuqi Zhou 1 , Predrag Radakovic 1 , Anjola Uprety 1 , Jun Xie 1 , Grant C. Flick 1
Affiliation
|
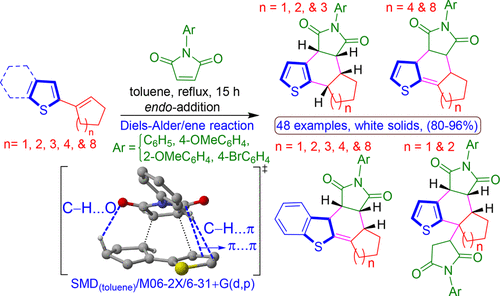
Scaffolds of thiophene and benzothiophene are the important class of bioactive compounds found abundant in nature. The Diels–Alder reactions of 2-(1′-cycloalkenyl)thiophenes and 2-(1′-cycloalkenyl)benzo[b]thiophenes having the alkene groups present in five-, six-, seven-, eight-, and twelve-membered rings with substituted N-phenylmaleimides are characterized. The size of the cycloalkene rings plays a critical role in dictating the product distributions of expected and isomerized Diels–Alder adducts. 2D NMR studies indicate that the isolated isomers for 2-(1′-cycloalkenyl)thiophenes having five-, six-, and seven-membered rings are aromatized benzothiophene products, whereas eight- and twelve-membered rings are un-rearranged adducts. In addition, the product of subsequent ene-reaction with the N-phenylmaleimide is isolated for the five- and six-membered ring cases. Interestingly, in the 2-(1′-cycloalkenyl)benzo[b]thiophene having five-, six-, seven-, eight-, and twelve-membered rings, the un-rearranged dibenzothiophene Diels–Alder adduct is isolated in every instance. Molecular mechanics and density functional theory (M06-2X and PBE0-D3) calculations are performed to understand the differential reactivity of the various dienes for both the initial Diels–Alder reaction and a possible, subsequent ene reaction.
中文翻译:

带有N-苯基马来酰亚胺的2-(1'-环烯基)噻吩和2-(1'-环烯基)苯并[ b ]噻吩的Diels-Alder / Ene反应性:环烯环大小对苯并噻吩和二苯并噻吩产物分布的作用
噻吩和苯并噻吩的支架是自然界中发现的丰富的生物活性化合物的重要类别。2-(1'-环烯基)噻吩和2-(1'-环烯基)苯并[ b ]噻吩的Diels-Alder反应具有在五个,六个,七个,八个和十二个烷基中存在的烯基N取代的成员环-苯基马来酰亚胺被表征。环烯环的大小在决定预期的和异构化的Diels-Alder加合物的产物分布中起着至关重要的作用。2D NMR研究表明,具有五,六和七元环的2-(1'-环烯基)噻吩的分离的异构体是芳构化的苯并噻吩产物,而八和十二元环是未重排的加合物。另外,对于五元和六元环的情况,分离出随后的与N-苯基马来酰亚胺的烯反应的产物。有趣的是,在2-(1'-环烯基)苯并[ b具有五元,六元,七元,八元和十二元环的]噻吩,在任何情况下均未分离未重排的二苯并噻吩Diels-Alder加合物。进行分子力学和密度泛函理论(M06-2X和PBE0-D3)的计算是为了了解各种二烯对于初始Diels-Alder反应和可能的后续烯反应的差异反应性。
更新日期:2020-04-24
中文翻译:

带有N-苯基马来酰亚胺的2-(1'-环烯基)噻吩和2-(1'-环烯基)苯并[ b ]噻吩的Diels-Alder / Ene反应性:环烯环大小对苯并噻吩和二苯并噻吩产物分布的作用
噻吩和苯并噻吩的支架是自然界中发现的丰富的生物活性化合物的重要类别。2-(1'-环烯基)噻吩和2-(1'-环烯基)苯并[ b ]噻吩的Diels-Alder反应具有在五个,六个,七个,八个和十二个烷基中存在的烯基N取代的成员环-苯基马来酰亚胺被表征。环烯环的大小在决定预期的和异构化的Diels-Alder加合物的产物分布中起着至关重要的作用。2D NMR研究表明,具有五,六和七元环的2-(1'-环烯基)噻吩的分离的异构体是芳构化的苯并噻吩产物,而八和十二元环是未重排的加合物。另外,对于五元和六元环的情况,分离出随后的与N-苯基马来酰亚胺的烯反应的产物。有趣的是,在2-(1'-环烯基)苯并[ b具有五元,六元,七元,八元和十二元环的]噻吩,在任何情况下均未分离未重排的二苯并噻吩Diels-Alder加合物。进行分子力学和密度泛函理论(M06-2X和PBE0-D3)的计算是为了了解各种二烯对于初始Diels-Alder反应和可能的后续烯反应的差异反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号