当前位置:
X-MOL 学术
›
Cancer Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide.
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-03-13 , DOI: 10.1002/cam4.2979 Koji Matsumoto 1 , Masato Takahashi 2 , Kazuhiko Sato 3 , Akihiko Osaki 4 , Toshimi Takano 5 , Yoichi Naito 6 , Kazuo Matsuura 7 , Kenjiro Aogi 8 , Kimiko Fujiwara 9 , Kenji Tamura 10 , Motoi Baba 11 , Shinya Tokunaga 12 , Gen Hirano 13 , Shigeru Imoto 14 , Chieko Miyazaki 15 , Kazuhiro Yanagihara 16 , Chiyo K Imamura 17 , Yasutaka Chiba 9 , Toshiaki Saeki 4
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-03-13 , DOI: 10.1002/cam4.2979 Koji Matsumoto 1 , Masato Takahashi 2 , Kazuhiko Sato 3 , Akihiko Osaki 4 , Toshimi Takano 5 , Yoichi Naito 6 , Kazuo Matsuura 7 , Kenjiro Aogi 8 , Kimiko Fujiwara 9 , Kenji Tamura 10 , Motoi Baba 11 , Shinya Tokunaga 12 , Gen Hirano 13 , Shigeru Imoto 14 , Chieko Miyazaki 15 , Kazuhiro Yanagihara 16 , Chiyo K Imamura 17 , Yasutaka Chiba 9 , Toshiaki Saeki 4
Affiliation
|
PURPOSE
To investigate whether palonosetron is better than granisetron in preventing chemotherapy-induced nausea and vomiting (CINV) in a three-drug combination with dexamethasone and fosaprepitant (Fos) in patients with breast cancer who are placed on anthracycline and cyclophosphamide (AC-based regimen).
PATIENTS AND METHODS
Chemo-naive women with primary breast cancer were randomly administered either palonosetron 0.75 mg (day 1) or granisetron 1 mg (day 1) combined with dexamethasone (12 mg at day 1, 8 mg at day 2 and day 3) and Fos 150 mg (day 1) before receiving AC-based regimen in a double-blind study. The primary endpoint was the complete response (CR) rate of emesis in cycle 1 in the delayed phase. This was defined as neither vomiting nor rescue drug usage for emesis at >24-120 hours after chemotherapy. Secondary endpoints were the CR in the acute/overall phase (0-24/0-120 hours, respectively, after chemotherapy), no nausea and vomiting, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE), and safety.
RESULTS
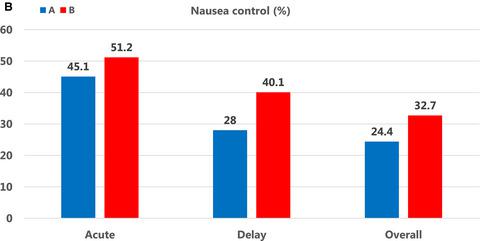
From December 2012 to October 2014, 326 patients were treated and evaluated (164/162 evaluable patients in granisetron/palonosetron arm, respectively). The CR during the delayed phase was 60.4% in the granisetron regimen and 62.3% in the palonosetron regimen. The CR during acute phase (73.2% vs 75.9%, respectively) and the CR during overall phase (54.9% in both regimens) were very identical. A significantly higher number of patients in the palonosetron arm were free from nausea during the delayed phase (28% vs 40.1%; P = .029). Adverse events were also identical, although infusion site reactions (ISR) were higher (20.3%-23.3%) than preceding studies in both regimens.
CONCLUSION
In combination with dexamethasone and Fos, this study suggests that palonosetron is not better than granisetron in chemo-naive patients with primary breast cancer receiving AC-based regimen. Administration of Fos in peripheral veins after AC-based regimen increased ISR.
中文翻译:

帕洛诺司琼与格拉司琼联合地塞米松和福沙匹坦预防接受蒽环类和环磷酰胺的乳腺癌患者化疗引起的恶心和呕吐的双盲、随机、多中心 3 期研究。
目的 研究帕洛诺司琼与地塞米松和福沙匹坦 (Fos) 三药联用在预防化疗引起的恶心和呕吐 (CINV) 方面是否优于格拉司琼,用于治疗接受蒽环类和环磷酰胺(基于 AC 的方案)的乳腺癌患者)。患者和方法 患有原发性乳腺癌的未接受过化疗的女性被随机给予帕洛诺司琼 0.75 mg(第 1 天)或格拉司琼 1 mg(第 1 天)联合地塞米松(第 1 天 12 mg,第 2 天和第 3 天 8 mg)和在一项双盲研究中,在接受基于 AC 的方案前服用 Fos 150 mg(第 1 天)。主要终点是延迟阶段第 1 周期呕吐的完全反应 (CR) 率。这被定义为在化疗后>24-120 小时既不呕吐也不使用急救药物治疗呕吐。次要终点是急性/整体阶段的 CR(化疗后分别为 0-24/0-120 小时)、无恶心和呕吐、不良事件通用术语标准 (PRO-CTCAE) 的患者报告结果版本, 和安全。结果 从 2012 年 12 月至 2014 年 10 月,共治疗和评估了 326 名患者(格拉司琼/帕洛诺司琼组分别为 164/162 名可评估患者)。格拉司琼方案延迟期的 CR 为 60.4%,帕洛诺司琼方案为 62.3%。急性期的 CR(分别为 73.2% 和 75.9%)和整个阶段的 CR(两种方案均为 54.9%)非常相同。在延迟阶段,帕洛诺司琼组中没有恶心的患者数量明显增多(28% 对 40.1%;P = .029)。不良事件也相同,尽管两种方案的输注部位反应 (ISR) 均高于先前的研究 (20.3%-23.3%)。结论 联合地塞米松和 Fos 的研究表明,帕洛诺司琼在接受基于 AC 方案的初治原发性乳腺癌患者中并不优于格拉司琼。基于 AC 的方案后在外周静脉中使用 Fos 会增加 ISR。
更新日期:2020-03-13
中文翻译:

帕洛诺司琼与格拉司琼联合地塞米松和福沙匹坦预防接受蒽环类和环磷酰胺的乳腺癌患者化疗引起的恶心和呕吐的双盲、随机、多中心 3 期研究。
目的 研究帕洛诺司琼与地塞米松和福沙匹坦 (Fos) 三药联用在预防化疗引起的恶心和呕吐 (CINV) 方面是否优于格拉司琼,用于治疗接受蒽环类和环磷酰胺(基于 AC 的方案)的乳腺癌患者)。患者和方法 患有原发性乳腺癌的未接受过化疗的女性被随机给予帕洛诺司琼 0.75 mg(第 1 天)或格拉司琼 1 mg(第 1 天)联合地塞米松(第 1 天 12 mg,第 2 天和第 3 天 8 mg)和在一项双盲研究中,在接受基于 AC 的方案前服用 Fos 150 mg(第 1 天)。主要终点是延迟阶段第 1 周期呕吐的完全反应 (CR) 率。这被定义为在化疗后>24-120 小时既不呕吐也不使用急救药物治疗呕吐。次要终点是急性/整体阶段的 CR(化疗后分别为 0-24/0-120 小时)、无恶心和呕吐、不良事件通用术语标准 (PRO-CTCAE) 的患者报告结果版本, 和安全。结果 从 2012 年 12 月至 2014 年 10 月,共治疗和评估了 326 名患者(格拉司琼/帕洛诺司琼组分别为 164/162 名可评估患者)。格拉司琼方案延迟期的 CR 为 60.4%,帕洛诺司琼方案为 62.3%。急性期的 CR(分别为 73.2% 和 75.9%)和整个阶段的 CR(两种方案均为 54.9%)非常相同。在延迟阶段,帕洛诺司琼组中没有恶心的患者数量明显增多(28% 对 40.1%;P = .029)。不良事件也相同,尽管两种方案的输注部位反应 (ISR) 均高于先前的研究 (20.3%-23.3%)。结论 联合地塞米松和 Fos 的研究表明,帕洛诺司琼在接受基于 AC 方案的初治原发性乳腺癌患者中并不优于格拉司琼。基于 AC 的方案后在外周静脉中使用 Fos 会增加 ISR。











































 京公网安备 11010802027423号
京公网安备 11010802027423号