当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective 1,4‐Reduction of Pyrimidin‐2‐ones to Synthesize Novel 3,4‐Dihydropyrimidin‐2(1H)‐ones Containing an Alkyl‐substituted Stereogenic Center
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-18 , DOI: 10.1002/ajoc.202000100 Fan‐Jie Meng 1 , Lei Shi 1 , Wen‐Feng Jiang 1 , Xiao‐Bing Lu 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-18 , DOI: 10.1002/ajoc.202000100 Fan‐Jie Meng 1 , Lei Shi 1 , Wen‐Feng Jiang 1 , Xiao‐Bing Lu 1
Affiliation
|
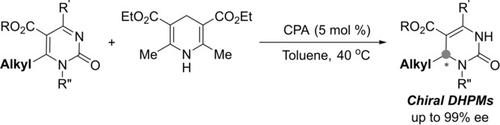
An efficient asymmetric 1,4‐reduction of pyrimidin‐2‐ones with a chiral phosphoric acid‐catalyzed system has been successfully developed, furnishing a series of chiral 3,4‐dihydro‐pyrimidin‐2‐one derivatives in excellent yields and enantioselectivities (up to 99% ee). Notably, this methodology enables a novel kind of chiral 3,4‐dihydropyrimidin‐2‐one with an alkyl stereogenic center to be prepared in high optical purity for the first time.
中文翻译:

对映选择性1,4-还原嘧啶-2-酮合成新型的3,4-二氢嘧啶-2(1H)-含有烷基取代的立体异构中心的化合物。
已经成功开发了一种有效的不对称的嘧啶-2-酮不对称的1,4-还原手性磷酸催化体系,提供了一系列具有优异收率和对映选择性的手性3,4-二氢嘧啶-2-酮衍生物(高达99%ee)。值得注意的是,这种方法可以首次以高光学纯度制备具有烷基立体异构中心的新型手性3,4-二氢嘧啶-2-酮。
更新日期:2020-03-18
中文翻译:

对映选择性1,4-还原嘧啶-2-酮合成新型的3,4-二氢嘧啶-2(1H)-含有烷基取代的立体异构中心的化合物。
已经成功开发了一种有效的不对称的嘧啶-2-酮不对称的1,4-还原手性磷酸催化体系,提供了一系列具有优异收率和对映选择性的手性3,4-二氢嘧啶-2-酮衍生物(高达99%ee)。值得注意的是,这种方法可以首次以高光学纯度制备具有烷基立体异构中心的新型手性3,4-二氢嘧啶-2-酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号