当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Cation Adsorption: Exploring Synergistic Effects on CO2 Electroreduction in Ionic Liquids
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-04-20 , DOI: 10.1002/celc.202000223 Alexander V. Rudnev 1, 2 , Kiran Kiran 1 , Peter Broekmann 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-04-20 , DOI: 10.1002/celc.202000223 Alexander V. Rudnev 1, 2 , Kiran Kiran 1 , Peter Broekmann 1
Affiliation
|
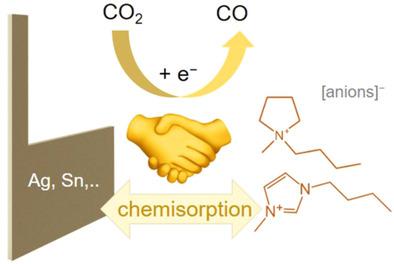
Ionic liquids (ILs) have gained attention as promising solvents as reaction media for the electrochemical conversion of CO2 into valued‐added products. ILs can co‐catalyze the CO2 reduction reaction (CO2RR), in particular, when Ag is applied as the primary metallic electrocatalyst. To broaden this co‐catalysis concept, we conducted a comprehensive study of a range of electrode materials exposed to three ILs, which differed in the chemical nature of their anions and cations. Here, we examine the activity of the electrode/IL combinations toward the CO2RR based on cyclic voltammetry data. The voltammetric experiments are complemented by gas chromatography analysis of the products, demonstrating that CO is the main product for most of the electrode/IL combinations. Our results demonstrate that the specific adsorption of the IL cation on the electrode plays a crucial role in the co‐catalytic effect of the ILs. Based on our analysis, we identify three classes of electrode materials, which differ in their synergistic interaction with the IL cations and their resulting CO2RR activity. The key criterion for this classification is the extent to which the CO2RR is superimposed on the reductive decomposition of the ILs.
中文翻译:

特定阳离子的吸附:探索离子液体中二氧化碳电还原的协同效应
离子液体(IL)作为有前途的溶剂已成为人们关注的溶剂,是将CO 2电化学转化为增值产品的反应介质。ILs可以共同催化CO 2还原反应(CO 2 RR),特别是在将Ag用作主要金属电催化剂时。为了拓宽这种共催化概念,我们对暴露于三种IL的一系列电极材料进行了全面研究,这三种ILs的阴离子和阳离子的化学性质不同。在这里,我们检查电极/ IL组合对CO 2的活性RR基于循环伏安数据。产物的气相色谱分析补充了伏安法实验,表明CO是大多数电极/ IL组合的主要产物。我们的结果表明,IL阳离子在电极上的特异性吸附在IL的共催化作用中起着至关重要的作用。根据我们的分析,我们确定了三类电极材料,它们在与IL阳离子的协同相互作用以及它们产生的CO 2 RR活性方面不同。该分类的关键标准是将CO 2 RR叠加在IL的还原分解上的程度。
更新日期:2020-04-22
中文翻译:

特定阳离子的吸附:探索离子液体中二氧化碳电还原的协同效应
离子液体(IL)作为有前途的溶剂已成为人们关注的溶剂,是将CO 2电化学转化为增值产品的反应介质。ILs可以共同催化CO 2还原反应(CO 2 RR),特别是在将Ag用作主要金属电催化剂时。为了拓宽这种共催化概念,我们对暴露于三种IL的一系列电极材料进行了全面研究,这三种ILs的阴离子和阳离子的化学性质不同。在这里,我们检查电极/ IL组合对CO 2的活性RR基于循环伏安数据。产物的气相色谱分析补充了伏安法实验,表明CO是大多数电极/ IL组合的主要产物。我们的结果表明,IL阳离子在电极上的特异性吸附在IL的共催化作用中起着至关重要的作用。根据我们的分析,我们确定了三类电极材料,它们在与IL阳离子的协同相互作用以及它们产生的CO 2 RR活性方面不同。该分类的关键标准是将CO 2 RR叠加在IL的还原分解上的程度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号