当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and SAR of Tetracyclic Inhibitors of Protein Kinase CK2 Derived from Furocarbazole W16.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-04-27 , DOI: 10.1002/cmdc.202000040 Lukas Kröger 1 , Constantin G Daniliuc 2 , Deeba Ensan 1 , Sebastian Borgert 1 , Christian Nienberg 1 , Miriam Lauwers 3 , Michaela Steinkrüger 3 , Joachim Jose 1 , Markus Pietsch 3 , Bernhard Wünsch 1, 4
ChemMedChem ( IF 3.6 ) Pub Date : 2020-04-27 , DOI: 10.1002/cmdc.202000040 Lukas Kröger 1 , Constantin G Daniliuc 2 , Deeba Ensan 1 , Sebastian Borgert 1 , Christian Nienberg 1 , Miriam Lauwers 3 , Michaela Steinkrüger 3 , Joachim Jose 1 , Markus Pietsch 3 , Bernhard Wünsch 1, 4
Affiliation
|
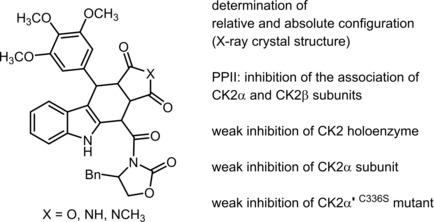
The serine/threonine kinase CK2 modulates the activity of more than 300 proteins and thus plays a crucial role in various physiological and pathophysiological processes including neurodegenerative disorders of the central nervous system and cancer. The enzymatic activity of CK2 is controlled by the equilibrium between the heterotetrameric holoenzyme CK2α2 β2 and its monomeric subunits CK2α and CK2β. A series of analogues of W16 ((3aR,4S,10S,10aS)-4-{[(S)-4-benzyl-2-oxo-1,3-oxazolidin-3-yl]carbonyl}-10-(3,4,5-trimethoxyphenyl)-4,5,10,10a-tetrahydrofuro[3,4-b]carbazole-1,3(3aH)-dione ((+)-3 a)) was prepared in an one-pot, three-component Levy reaction. The stereochemistry of the tetracyclic compounds was analyzed. Additionally, the chemically labile anhydride structure of the furocarbazoles 3 was replaced by a more stable imide (9) and N-methylimide (10) substructure. The enantiomer (-)-3 a (Ki =4.9 μM) of the lead compound (+)-3 a (Ki =31 μM) showed a more than sixfold increased inhibition of the CK2α/CK2β interaction (protein-protein interaction inhibition, PPII) in a microscale thermophoresis (MST) assay. However, (-)-3 a did not show an increased enzyme inhibition of the CK2α2 β2 holoenzyme, the CK2α subunit or the mutated CK2α' C336S subunit in the capillary electrophoresis assay. In the pyrrolocarbazole series, the imide (-)-9 a (Ki =3.6 μM) and the N-methylimide (+)-10 a (Ki =2.8 μM) represent the most promising inhibitors of the CK2α/CK2β interaction. However, neither compound could inhibit enzymatic activity. Unexpectedly, the racemic tetracyclic pyrrolocarbazole (±)-12, with a carboxy moiety in the 4-position, displays the highest CK2α/CK2β interaction inhibition (Ki =1.8 μM) of this series of compounds.
中文翻译:

呋喃咔唑W16衍生的蛋白激酶CK2四环抑制剂的合成和SAR。
丝氨酸/苏氨酸激酶CK2调节300多种蛋白质的活性,因此在各种生理和病理生理过程(包括中枢神经系统的神经退行性疾病和癌症)中起着至关重要的作用。CK2的酶活性由异四聚体全酶CK2α2β2与其单体亚基CK2α和CK2β之间的平衡控制。W16((3aR,4S,10S,10aS)-4-{[((S)-4-苄基-2-氧代-1,3-恶唑烷-3-基]羰基}羰基} -10-(3的一系列类似物一锅制备1,4,5-三甲氧基苯基)-4,5,10,10a-四氢呋喃[3,4-b]咔唑-1,3(3aH)-二酮((+)-3 a)) ,三成分征税反应。分析了四环化合物的立体化学。另外,呋喃咔唑3的化学不稳定的酸酐结构被更稳定的酰亚胺(9)和N-甲基酰亚胺(10)子结构所取代。铅化合物(+)-3 a(Ki = 31μM)的对映体(-)-3 a(Ki = 4.9μM)显示出对CK2α/CK2β相互作用的抑制作用(蛋白质-蛋白质相互作用抑制, PPII)。但是,(-)-3a在毛细管电泳分析中并未显示出对CK2α2β2全酶,CK2α亚基或突变的CK2α'C336S亚基的酶抑制作用增加。在吡咯并咔唑系列中,酰亚胺(-)-9 a(Ki = 3.6μM)和N-甲基酰亚胺(+)-10 a(Ki = 2.8μM)代表CK2α/CK2β相互作用的最有希望的抑制剂。但是,两种化合物都不能抑制酶活性。不料,
更新日期:2020-03-13
中文翻译:

呋喃咔唑W16衍生的蛋白激酶CK2四环抑制剂的合成和SAR。
丝氨酸/苏氨酸激酶CK2调节300多种蛋白质的活性,因此在各种生理和病理生理过程(包括中枢神经系统的神经退行性疾病和癌症)中起着至关重要的作用。CK2的酶活性由异四聚体全酶CK2α2β2与其单体亚基CK2α和CK2β之间的平衡控制。W16((3aR,4S,10S,10aS)-4-{[((S)-4-苄基-2-氧代-1,3-恶唑烷-3-基]羰基}羰基} -10-(3的一系列类似物一锅制备1,4,5-三甲氧基苯基)-4,5,10,10a-四氢呋喃[3,4-b]咔唑-1,3(3aH)-二酮((+)-3 a)) ,三成分征税反应。分析了四环化合物的立体化学。另外,呋喃咔唑3的化学不稳定的酸酐结构被更稳定的酰亚胺(9)和N-甲基酰亚胺(10)子结构所取代。铅化合物(+)-3 a(Ki = 31μM)的对映体(-)-3 a(Ki = 4.9μM)显示出对CK2α/CK2β相互作用的抑制作用(蛋白质-蛋白质相互作用抑制, PPII)。但是,(-)-3a在毛细管电泳分析中并未显示出对CK2α2β2全酶,CK2α亚基或突变的CK2α'C336S亚基的酶抑制作用增加。在吡咯并咔唑系列中,酰亚胺(-)-9 a(Ki = 3.6μM)和N-甲基酰亚胺(+)-10 a(Ki = 2.8μM)代表CK2α/CK2β相互作用的最有希望的抑制剂。但是,两种化合物都不能抑制酶活性。不料,









































 京公网安备 11010802027423号
京公网安备 11010802027423号