当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development and Structural Evaluation of N-Alkylated trans-2-Phenylcyclopropylamine-Based LSD1 Inhibitors.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-03-30 , DOI: 10.1002/cmdc.202000014 Hideaki Niwa 1, 2, 3 , Shin Sato 1, 2, 3 , Noriko Handa 1 , Toru Sengoku 1, 4 , Takashi Umehara 1, 2, 3 , Shigeyuki Yokoyama 1, 4, 5
ChemMedChem ( IF 3.6 ) Pub Date : 2020-03-30 , DOI: 10.1002/cmdc.202000014 Hideaki Niwa 1, 2, 3 , Shin Sato 1, 2, 3 , Noriko Handa 1 , Toru Sengoku 1, 4 , Takashi Umehara 1, 2, 3 , Shigeyuki Yokoyama 1, 4, 5
Affiliation
|
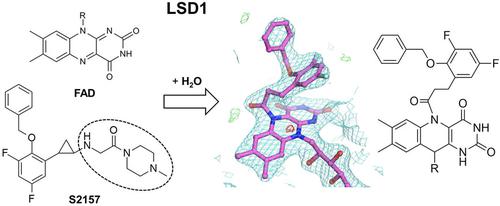
Lysine-specific demethylase 1 (LSD1) is a flavin adenine dinucleotide (FAD)-dependent enzyme that catalyzes the demethylation of histone H3 and regulates gene expression. Because it is implicated in the regulation of diseases such as acute myeloid leukemia, potent LSD1-specific inhibitors have been pursued. Trans-2-phenylcyclopropylamine (2-PCPA)-based inhibitors featuring substitutions on the amino group have emerged, with sub-micromolar affinities toward LSD1 and high selectivities over monoamine oxidases (MAOs). We synthesized two N-alkylated 2-PCPA-based LSD1 inhibitors, S2116 and S2157, based on the previously developed S2101. S2116 and S2157 exhibited enhanced potency for LSD1 by 2.0- to 2.6-fold, as compared with S2101. In addition, they exhibited improved selectivity over MAOs. Structural analyses of LSD1 co-crystallized with S2101, S2116, S2157, or another N-alkylated inhibitor (FCPA-MPE) confirmed that the N-substituents enhance the potency of a 2-PCPA-based inhibitor of LSD1, without constituting the adduct formed with FAD.
中文翻译:

N-烷基化的反式-2-苯基环丙胺基LSD1抑制剂的开发和结构评价。
赖氨酸特异性脱甲基酶1(LSD1)是黄素腺嘌呤二核苷酸(FAD)依赖性酶,可催化组蛋白H3的脱甲基化并调节基因表达。由于它与疾病的调控有关,例如急性髓细胞性白血病,因此一直在寻求有效的LSD1特异性抑制剂。以氨基取代为特征的基于反式-2-苯基环丙胺(2-PCPA)的抑制剂已经出现,对LSD1具有亚微摩尔亲和力,对单胺氧化酶(MAO)具有高选择性。基于以前开发的S2101,我们合成了两种基于N-烷基化的2-PCPA的LSD1抑制剂S2116和S2157。与S2101相比,S2116和S2157对LSD1的效力提高了2.0到2.6倍。另外,它们表现出比MAO更高的选择性。与S2101,S2116共结晶的LSD1的结构分析
更新日期:2020-03-30
中文翻译:

N-烷基化的反式-2-苯基环丙胺基LSD1抑制剂的开发和结构评价。
赖氨酸特异性脱甲基酶1(LSD1)是黄素腺嘌呤二核苷酸(FAD)依赖性酶,可催化组蛋白H3的脱甲基化并调节基因表达。由于它与疾病的调控有关,例如急性髓细胞性白血病,因此一直在寻求有效的LSD1特异性抑制剂。以氨基取代为特征的基于反式-2-苯基环丙胺(2-PCPA)的抑制剂已经出现,对LSD1具有亚微摩尔亲和力,对单胺氧化酶(MAO)具有高选择性。基于以前开发的S2101,我们合成了两种基于N-烷基化的2-PCPA的LSD1抑制剂S2116和S2157。与S2101相比,S2116和S2157对LSD1的效力提高了2.0到2.6倍。另外,它们表现出比MAO更高的选择性。与S2101,S2116共结晶的LSD1的结构分析











































 京公网安备 11010802027423号
京公网安备 11010802027423号