当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microwave assisted synthesis, docking and antimalarial evaluation of hybrid PABA‐substituted 1,3,5‐triazine derivatives
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-15 , DOI: 10.1002/jhet.3955 Nayana Adhikari 1 , Ankita Kashyap 1 , Anshul Shakya 1 , Surajit Kumar Ghosh 1 , Dibya Ranjan Bhattacharyya 2 , Hans Raj Bhat 1 , Udaya Pratap Singh 3
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-15 , DOI: 10.1002/jhet.3955 Nayana Adhikari 1 , Ankita Kashyap 1 , Anshul Shakya 1 , Surajit Kumar Ghosh 1 , Dibya Ranjan Bhattacharyya 2 , Hans Raj Bhat 1 , Udaya Pratap Singh 3
Affiliation
|
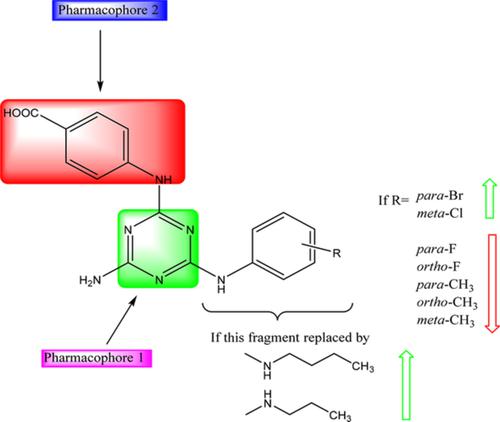
A series of novel PABA‐substituted 1,3,5‐triazine derivatives were developed via microwave assisted synthesis and subsequently tested for antimalarial activity against chloroquine sensitive 3D7 strain of Plasmodium falciparum using chloroquine as standard. Antimalarial screening result showed that synthesized compounds exhibited IC50 in the range of 4.46 to 79.72 μg mL−1. Among the tested compounds, 4c and 4f showed significant antimalarial activity with low binding energies (BE) ‐172.32 and 160.41 kcal mol−1 via interacting with Arg122 through the involvement of COOH of the phenyl linked to 1,3,5‐triazine. In conclusion, these core scaffolds can be used for future antimalarial drug development.
中文翻译:

微波辅助合成,对接和杂化PABA取代的1,3,5-三嗪衍生物的抗疟疾评估
通过微波辅助合成开发了一系列新颖的PABA取代的1,3,5-三嗪衍生物,随后使用氯喹作为标准品,测试了其对恶性疟原虫对氯喹敏感的3D7菌株的抗疟活性。抗疟疾筛查结果表明,合成化合物的IC 50为4.46至79.72μgmL -1。在测试的化合物中,4c和4f表现出显着的抗疟活性,且结合能(BE)低-172.32和160.41 kcal mol -1通过与1,3,5-三嗪连接的苯基的COOH参与与Arg122相互作用。总之,这些核心支架可用于未来的抗疟药物开发。
更新日期:2020-03-15
中文翻译:

微波辅助合成,对接和杂化PABA取代的1,3,5-三嗪衍生物的抗疟疾评估
通过微波辅助合成开发了一系列新颖的PABA取代的1,3,5-三嗪衍生物,随后使用氯喹作为标准品,测试了其对恶性疟原虫对氯喹敏感的3D7菌株的抗疟活性。抗疟疾筛查结果表明,合成化合物的IC 50为4.46至79.72μgmL -1。在测试的化合物中,4c和4f表现出显着的抗疟活性,且结合能(BE)低-172.32和160.41 kcal mol -1通过与1,3,5-三嗪连接的苯基的COOH参与与Arg122相互作用。总之,这些核心支架可用于未来的抗疟药物开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号