Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Platelet‐derived microvesicles promote endothelial progenitor cell proliferation in intimal injury by delivering TGF‐β1
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-03-13 , DOI: 10.1111/febs.15293 Jing Yan 1 , Han Bao 1 , Yang‐Jing Fan 1 , Zong‐Lai Jiang 1 , Ying‐Xin Qi 1 , Yue Han 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-03-13 , DOI: 10.1111/febs.15293 Jing Yan 1 , Han Bao 1 , Yang‐Jing Fan 1 , Zong‐Lai Jiang 1 , Ying‐Xin Qi 1 , Yue Han 1
Affiliation
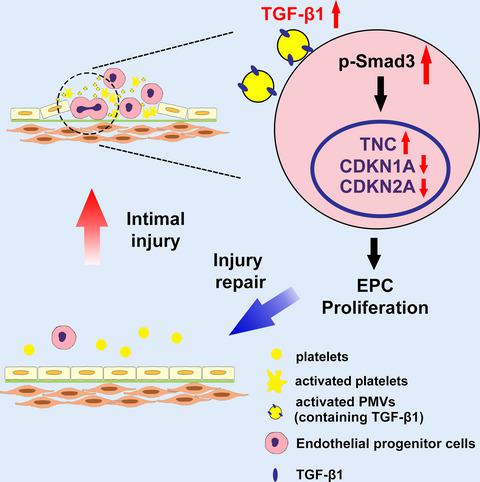
|
Intimal injury is an early stage of several cardiovascular diseases. Endothelial progenitor cells (EPCs) play a significant role in endothelial repair following vascular injury. Once the intima is damaged, EPCs are mobilized from the bone marrow to the injury site. Meanwhile, the injury to the intimal surface triggers platelet degranulation, aggregation, and adhesion to the damaged endothelium, and exposed collagen stimulates platelet to secrete platelet‐derived microvesicles (PMVs). However, the role of PMVs in EPC function during this process remains unknown. In an in vivo study, EPCs and platelets were found to adhere to the injury site in Sprague‐Dawley (SD) rat vascular injury model. In vitro, collagen stimulation induced the release of PMVs, and collagen‐activated PMVs (ac.PMVs) significantly promoted EPC proliferation. Transforming growth factor‐β1 (TGF‐β1) content was increased in ac.PMVs. Activated PMVs significantly upregulated Smad3 phosphorylation in EPCs and increased Smad3 nuclear translocation from the cytoplasm. TGF‐β1 knockdown ac.PMVs downregulated EPC proliferation. Recombinant TGF‐β1 enhanced EPC proliferation. The TGF‐β1 inhibitor SB431542 significantly repressed the intracellular signal triggered by ac.PMVs. Furthermore, the Smad3‐specific phosphorylation inhibitor SIS3 effectively reversed the cell proliferation induced by ac.PMVs. Smad3 translocated to the nucleus and enhanced EPC proliferation via its downstream genes tenascin C (TNC), CDKN1A, and CDKN2A. r‐TGF‐β1 promoted reendothelialization and EPC proliferation in vivo. Our data demonstrate that activated PMVs deliver TGF‐β1 from collagen‐activated platelets to EPCs, which in turn activates Smad3 phosphorylation and regulates TNC, CDKN1A, and CDKN2A expression to promote EPC proliferation, suggesting that PMVs act as a key transporter and a potential therapeutic target for vascular injury.
中文翻译:

血小板衍生的微泡通过递送TGF-β1促进内膜损伤中的内皮祖细胞增殖
内膜损伤是几种心血管疾病的早期阶段。内皮祖细胞(EPC)在血管损伤后的内皮修复中起重要作用。一旦内膜受损,EPCs从骨髓动员到损伤部位。同时,对内膜表面的损伤会触发血小板脱粒,聚集和粘附于受损的内皮,暴露的胶原蛋白会刺激血小板分泌源自血小板的微囊泡(PMV)。但是,在此过程中PMV在EPC功能中的作用仍然未知。在体内研究中,在Sprague-Dawley(SD)大鼠血管损伤模型中,发现EPC和血小板粘附在损伤部位。体外,胶原蛋白刺激诱导了PMV的释放,而胶原蛋白激活的PMV(ac.PMVs)则显着促进了EPC的增殖。ac.PMVs中转化生长因子-β1(TGF-β1)含量增加。激活的PMV显着上调了EPC中的Smad3磷酸化并增加了Smad3从细胞质中的核易位。TGF-β1敲低的ac.PMVs下调了EPC增殖。重组TGF-β1增强EPC增殖。TGF-β1抑制剂SB431542可显着抑制ac.PMV触发的细胞内信号。此外,Smad3特异性磷酸化抑制剂SIS3有效逆转了由ac.PMV诱导的细胞增殖。Smad3通过其下游基因Tenascin C(TNC),CDKN1A和CDKN2A易位至细胞核并增强EPC增殖。r-TGF-β1促进内皮细胞再生和EPC增殖体内。我们的数据表明,激活的PMV将TGF-β1从胶原蛋白激活的血小板传递给EPC,进而激活Smad3磷酸化并调节TNC,CDKN1A和CDKN2A的表达,从而促进EPC增殖,这表明PMVs是关键的转运蛋白和潜在的治疗剂血管损伤的目标。
更新日期:2020-03-13
中文翻译:

血小板衍生的微泡通过递送TGF-β1促进内膜损伤中的内皮祖细胞增殖
内膜损伤是几种心血管疾病的早期阶段。内皮祖细胞(EPC)在血管损伤后的内皮修复中起重要作用。一旦内膜受损,EPCs从骨髓动员到损伤部位。同时,对内膜表面的损伤会触发血小板脱粒,聚集和粘附于受损的内皮,暴露的胶原蛋白会刺激血小板分泌源自血小板的微囊泡(PMV)。但是,在此过程中PMV在EPC功能中的作用仍然未知。在体内研究中,在Sprague-Dawley(SD)大鼠血管损伤模型中,发现EPC和血小板粘附在损伤部位。体外,胶原蛋白刺激诱导了PMV的释放,而胶原蛋白激活的PMV(ac.PMVs)则显着促进了EPC的增殖。ac.PMVs中转化生长因子-β1(TGF-β1)含量增加。激活的PMV显着上调了EPC中的Smad3磷酸化并增加了Smad3从细胞质中的核易位。TGF-β1敲低的ac.PMVs下调了EPC增殖。重组TGF-β1增强EPC增殖。TGF-β1抑制剂SB431542可显着抑制ac.PMV触发的细胞内信号。此外,Smad3特异性磷酸化抑制剂SIS3有效逆转了由ac.PMV诱导的细胞增殖。Smad3通过其下游基因Tenascin C(TNC),CDKN1A和CDKN2A易位至细胞核并增强EPC增殖。r-TGF-β1促进内皮细胞再生和EPC增殖体内。我们的数据表明,激活的PMV将TGF-β1从胶原蛋白激活的血小板传递给EPC,进而激活Smad3磷酸化并调节TNC,CDKN1A和CDKN2A的表达,从而促进EPC增殖,这表明PMVs是关键的转运蛋白和潜在的治疗剂血管损伤的目标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号