当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantifying Protein-Protein Interactions by Molecular Counting with Mass Photometry.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-13 , DOI: 10.1002/anie.202001578 Fabian Soltermann 1 , Eric D B Foley 1 , Veronica Pagnoni 1 , Martin Galpin 1 , Justin L P Benesch 1 , Philipp Kukura 1 , Weston B Struwe 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-13 , DOI: 10.1002/anie.202001578 Fabian Soltermann 1 , Eric D B Foley 1 , Veronica Pagnoni 1 , Martin Galpin 1 , Justin L P Benesch 1 , Philipp Kukura 1 , Weston B Struwe 1
Affiliation
|
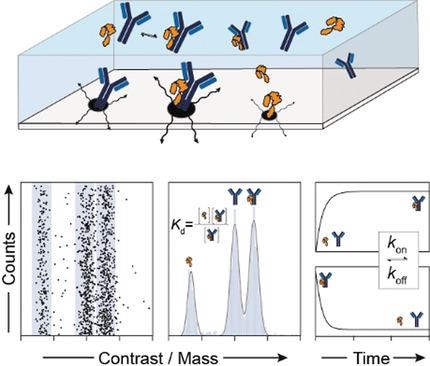
Interactions between biomolecules control the processes of life in health and their malfunction in disease, making their characterization and quantification essential. Immobilization‐ and label‐free analytical techniques are desirable because of their simplicity and minimal invasiveness, but they struggle with quantifying tight interactions. Here, we show that mass photometry can accurately count, distinguish by molecular mass, and thereby reveal the relative abundances of different unlabelled biomolecules and their complexes in mixtures at the single‐molecule level. These measurements determine binding affinities over four orders of magnitude at equilibrium for both simple and complex stoichiometries within minutes, as well as the associated kinetics. These results introduce mass photometry as a rapid, simple and label‐free method for studying sub‐micromolar binding affinities, with potential for extension towards a universal approach for characterizing complex biomolecular interactions.
中文翻译:

通过分子计数和质量光度测定定量蛋白质-蛋白质相互作用。
生物分子之间的相互作用控制着健康的生命过程及其在疾病中的功能障碍,因此对其进行表征和量化至关重要。无固定化和无标记的分析技术因其简单性和微创性而受到欢迎,但它们难以量化紧密的相互作用。在这里,我们证明质量光度法可以准确计数,通过分子质量进行区分,从而在单分子水平上揭示混合物中不同未标记生物分子及其复合物的相对丰度。这些测量可在几分钟内确定简单和复杂化学计量的平衡状态下四个数量级的结合亲和力以及相关的动力学。这些结果将质量光度测定法作为一种快速、简单且无标记的方法来研究亚微摩尔结合亲和力,并有可能扩展到表征复杂生物分子相互作用的通用方法。
更新日期:2020-03-13
中文翻译:

通过分子计数和质量光度测定定量蛋白质-蛋白质相互作用。
生物分子之间的相互作用控制着健康的生命过程及其在疾病中的功能障碍,因此对其进行表征和量化至关重要。无固定化和无标记的分析技术因其简单性和微创性而受到欢迎,但它们难以量化紧密的相互作用。在这里,我们证明质量光度法可以准确计数,通过分子质量进行区分,从而在单分子水平上揭示混合物中不同未标记生物分子及其复合物的相对丰度。这些测量可在几分钟内确定简单和复杂化学计量的平衡状态下四个数量级的结合亲和力以及相关的动力学。这些结果将质量光度测定法作为一种快速、简单且无标记的方法来研究亚微摩尔结合亲和力,并有可能扩展到表征复杂生物分子相互作用的通用方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号