当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Purification, characterization and molecular cloning of a dicaffeoylquinic acid-hydrolyzing esterase from human-derived Lactobacillus fermentum LF-12.
Food & Function ( IF 5.1 ) Pub Date : 2020-04-30 , DOI: 10.1039/d0fo00029a Yujin Liu 1 , Minhao Xie 2 , Peng Wan 1 , Guijie Chen 1 , Chunxu Chen 1 , Dan Chen 1 , Shijie Yu 1 , Xiaoxiong Zeng 1 , Yi Sun 1
Food & Function ( IF 5.1 ) Pub Date : 2020-04-30 , DOI: 10.1039/d0fo00029a Yujin Liu 1 , Minhao Xie 2 , Peng Wan 1 , Guijie Chen 1 , Chunxu Chen 1 , Dan Chen 1 , Shijie Yu 1 , Xiaoxiong Zeng 1 , Yi Sun 1
Affiliation
|
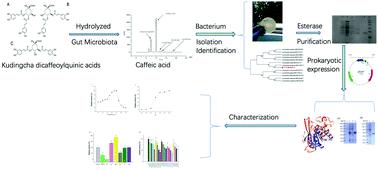
Dicaffeoylquinic acids (DiCQAs), the main components of kudingcha made from the leaves of Ilex kudingcha, could be transformed by gut microbiota. However, the information about the related microorganisms and enzymes involved in the biotransformation of DiCQAs in the human gut is limited. Therefore, a strain of bacteria that could hydrolyze DiCQAs, belonging to Lactobacillus fermentum named L. fermentum LF-12, was isolated from human feces in the present study. Furthermore, an esterase for the hydrolysis of DiCQAs was purified from L. fermentum LF-12 and heterogeneously expressed in Escherichia coli. The esterase could be induced to exert superior hydrolytic activity in the presence of lactose as the carbon source. The molecular weight of the purified esterase was determined to be 31.9 kDa, and the isoelectric point, optimal pH and temperature for the esterase were 4.71, 6.5 and 45 °C, respectively. The enzyme activity was improved by Mg2+ and Ca2+, and reduced by Co2+, Cu2+, EDTA and some kinds of organic solvents. The present results provide new insights into the metabolism of DiCQAs by the human gut.
中文翻译:

人源发酵乳杆菌LF-12中二咖啡酰奎宁酸水解酯酶的纯化,鉴定和分子克隆。
由苦丁茶的叶子制成的苦丁茶的主要成分二咖啡酰奎尼酸(DiCQAs)可以通过肠道菌群转化。但是,有关DiCQA在人肠道中生物转化的相关微生物和酶的信息是有限的。因此,在本研究中,从人粪便中分离出了一种可水解DiCQAs的细菌菌株,该菌株属于发酵乳杆菌LF-12。此外,从发酵乳杆菌LF-12中纯化了用于水解DiCQA的酯酶,并在大肠杆菌中异源表达。在乳糖作为碳源的情况下,可以诱导酯酶发挥出色的水解活性。纯化的酯酶的分子量确定为31.9 kDa,等电点为 酯酶的最佳pH和温度分别为4.71、6.5和45°C。Mg2 +和Ca2 +提高了酶的活性,Co2 +,Cu2 +,EDTA和某些有机溶剂降低了酶的活性。目前的结果提供了新的见解,对人类肠内DiCQA的代谢。
更新日期:2020-03-13
中文翻译:

人源发酵乳杆菌LF-12中二咖啡酰奎宁酸水解酯酶的纯化,鉴定和分子克隆。
由苦丁茶的叶子制成的苦丁茶的主要成分二咖啡酰奎尼酸(DiCQAs)可以通过肠道菌群转化。但是,有关DiCQA在人肠道中生物转化的相关微生物和酶的信息是有限的。因此,在本研究中,从人粪便中分离出了一种可水解DiCQAs的细菌菌株,该菌株属于发酵乳杆菌LF-12。此外,从发酵乳杆菌LF-12中纯化了用于水解DiCQA的酯酶,并在大肠杆菌中异源表达。在乳糖作为碳源的情况下,可以诱导酯酶发挥出色的水解活性。纯化的酯酶的分子量确定为31.9 kDa,等电点为 酯酶的最佳pH和温度分别为4.71、6.5和45°C。Mg2 +和Ca2 +提高了酶的活性,Co2 +,Cu2 +,EDTA和某些有机溶剂降低了酶的活性。目前的结果提供了新的见解,对人类肠内DiCQA的代谢。











































 京公网安备 11010802027423号
京公网安备 11010802027423号